Low rates of nucleoside reverse transcriptase inhibitor resistance in a well-monitored cohort in South Africa on antiretroviral therapy
- PMID: 22293461
- PMCID: PMC3600633
- DOI: 10.3851/IMP1985
Low rates of nucleoside reverse transcriptase inhibitor resistance in a well-monitored cohort in South Africa on antiretroviral therapy
Abstract
Background: The emergence of complex HIV-1 drug resistance mutations has been linked to the duration of time patients are on a failing antiretroviral drug regimen. This study reports on resistance profiles in a closely monitored subtype C infected cohort.
Methods: A total of 812 participants were enrolled into the CIPRA-SA 'safeguard the household' study, viral loads were determined at 12-weekly intervals for 96 weeks. Virological failure was defined as either a <1.5 log decrease in viral load at week 12 or two consecutive viral load measurements of >1,000 RNA copies/ml after week 24. Regimens prescribed were in line with the South African roll-out programme (stavudine, lamivudine, efavirenz or nevirapine). Viral RNA was extracted from patients with virological failure, and pol reverse-transcriptase PCR and sequence analysis were performed to determine drug-resistant mutations.
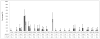
Results: Virological failure was observed in 83 participants on the first-line regimen during the study period, of which 61 (73%) had HIV-1 drug-resistant mutations. The M184V mutation was the most frequent (n=46; 65%), followed by K103N (46%) and Y181C (21%). Thymidine analogue mutations were infrequent (1%) and Q151M was not observed.
Conclusions: Drug resistance profiles were less complex than has been previously reported in South Africa using the same antiretroviral drug regimens. These data suggest that frequent viral load monitoring limits the level and complexity of resistance observed in HIV-1 subtype C, preserving susceptibility to second-line options.
Conflict of interest statement
Figures


References
-
- Hosseinipour MC, van Oosterhout JJ, Weigel R, Phiri S, Kamwendo D, Parkin N, Fiscus SA, Nelson JA, Eron JJ, Kumwenda J. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–1134. - PMC - PubMed
-
- Hosseinipour MC, Kumwenda JJ, Weigel R, Brown LB, Mzinganjira D, Mhango B, Eron JJ, Phiri S, van Oosterhout JJ. Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med. 2010;11:510–518. - PMC - PubMed
-
- Marconi VC, Sunpath H, Lu Z, Gordon M, Koranteng-Apeagyei K, Hampton J, Carpenter S, Giddy J, Ross D, Holst H, Losina E, Walker BD, Kuritzkes DR. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46:1589–1597. - PMC - PubMed
-
- Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;53:480–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

