Role of macrophages in mobilization of hematopoietic progenitor cells from bone marrow after hemorrhagic shock
- PMID: 22293600
- PMCID: PMC3328610
- DOI: 10.1097/SHK.0b013e318249b81d
Role of macrophages in mobilization of hematopoietic progenitor cells from bone marrow after hemorrhagic shock
Abstract
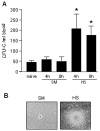
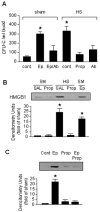
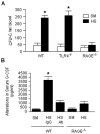
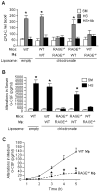
The release of hematopoietic progenitor cells (HPCs) from bone marrow (BM) is under tight homeostatic control. Under stress conditions, HPCs migrate from BM and egress into circulation to participate in immune response, wound repair, or tissue regeneration. Hemorrhagic shock with resuscitation (HS/R), resulting from severe trauma and major surgery, promotes HPC mobilization from BM, which, in turn, affects post-HS immune responses. In this study, we investigated the mechanism of HS/R regulation of HPC mobilization from BM. Using a mouse HS/R model, we demonstrate that the endogenous alarmin molecule high-mobility group box 1 mediates HS/R-induced granulocyte colony-stimulating factor secretion from macrophages (Mϕ in a RAGE [receptor for advanced glycation end products] signaling-dependent manner. Secreted granulocyte colony-stimulating factor, in turn, induces HPC egress from BM. We also show that activation of β-adrenergic receptors on Mϕ by catecholamine mediates the HS/R-induced release of high-mobility group box 1. These data indicate that HS/R, a global ischemia-reperfusion stimulus, regulates HPC mobilization through a series of interacting pathways that include neuroendocrine and innate immune systems, in which Mϕ play a central role.
Figures
References
-
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. - PubMed
-
- Hannoush EJ, Sifri ZC, Elhassan IO, Mohr AM, Alzate WD, Offin M, Livingston DH. Impact of enhanced mobilization of bone marrow derived cells to site of injury. J Trauma. 2011;71:283–289. discussion 289–291. - PubMed
-
- Shah S, Ulm J, Sifri ZC, Mohr AM, Livingston DH. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009;67:315–321. discussion 321–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

