Safety of embolic protection device-assisted and unprotected intravascular ultrasound in evaluating carotid artery atherosclerotic lesions
- PMID: 22293887
- PMCID: PMC3560589
- DOI: 10.12659/msm.882452
Safety of embolic protection device-assisted and unprotected intravascular ultrasound in evaluating carotid artery atherosclerotic lesions
Abstract
Background: Significant atherosclerotic stenosis of internal carotid artery (ICA) origin is common (5-10% at ≥ 60 years). Intravascular ultrasound (IVUS) enables high-resolution (120 µm) plaque imaging, and IVUS-elucidated features of the coronary plaque were recently shown to be associated with its symptomatic rupture/thrombosis risk. Safety of the significant carotid plaque IVUS imaging in a large unselected population is unknown.
Material/methods: We prospectively evaluated the safety of embolic protection device (EPD)-assisted vs. unprotected ICA-IVUS in a series of consecutive subjects with ≥ 50% ICA stenosis referred for carotid artery stenting (CAS), including 104 asymptomatic (aS) and 187 symptomatic (S) subjects (age 47-83 y, 187 men). EPD use was optional for IVUS, but mandatory for CAS.
Results: Evaluation was performed of 107 ICAs (36.8%) without EPD and 184 with EPD. Lesions imaged under EPD were overall more severe (peak-systolic velocity 2.97 ± 0.08 vs. 2.20 ± 0.08 m/s, end-diastolic velocity 1.0 ± 0.04 vs. 0.7 ± 0.03 m/s, stenosis severity of 85.7 ± 0.5% vs. 77.7 ± 0.6% by catheter angiography; mean ± SEM; p<0.01 for all comparisons) and more frequently S (50.0% vs. 34.6%, p=0.01). No ICA perforation or dissection, and no major stroke or death occurred. There was no IVUS-triggered cerebral embolization. In the procedures of (i) unprotected IVUS and no CAS, (ii) unprotected IVUS followed by CAS (filters - 39, flow reversal/blockade - 3), (iii) EPD-protected (filters - 135, flow reversal/blockade - 48) IVUS + CAS, TIA occurred in 1.5% vs. 4.8% vs. 2.7%, respectively, and minor stroke in 0% vs. 2.4% vs. 2.1%, respectively. EPD intolerance (on-filter ICA spasm or flow reversal/blockade intolerance) occurred in 9/225 (4.0%). IVUS increased the procedure duration by 7.27 ± 0.19 min.
Conclusions: Carotid IVUS is safe and, for the less severe lesions in particular, it may not require mandatory EPD use. High-risk lesions can be safely evaluated with IVUS under flow reversal/blockade.
Figures

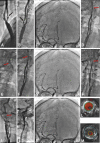

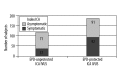
Similar articles
-
Atherosclerotic plaque composition assessed by virtual histology intravascular ultrasound and cerebral embolization after carotid stenting.J Vasc Surg. 2010 Nov;52(5):1188-94. doi: 10.1016/j.jvs.2010.05.101. Epub 2010 Jul 23. J Vasc Surg. 2010. PMID: 20655687
-
Carotid artery stenting according to the tailored-CAS algorithm is associated with a low complication rate at 30 days: data from the TARGET-CAS study.Kardiol Pol. 2012;70(4):378-86. Kardiol Pol. 2012. PMID: 22528713
-
A Microscopic and Biomarker Evaluation of Embolic Filter Debris Collected During Carotid Artery Stenting.J Endovasc Ther. 2016 Apr;23(2):275-84. doi: 10.1177/1526602816628284. Epub 2016 Feb 2. J Endovasc Ther. 2016. PMID: 26839124
-
Evidence overview: benefit of cerebral protection devices during carotid artery stenting.J Cardiovasc Surg (Torino). 2017 Apr;58(2):170-177. doi: 10.23736/S0021-9509.16.09848-7. Epub 2016 Dec 22. J Cardiovasc Surg (Torino). 2017. PMID: 28004899 Review.
-
Stenting for Internal Carotid Artery Stenosis Associated with Persistent Primitive Hypoglossal Artery Using Proximal Flow Blockade and Distal Protection System: A Technical Case Report and Literature Review.J Stroke Cerebrovasc Dis. 2016 Jun;25(6):e98-e102. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.026. Epub 2016 Apr 19. J Stroke Cerebrovasc Dis. 2016. PMID: 27105567 Review.
Cited by
-
Imaging modality-dependent carotid stenosis severity variations against intravascular ultrasound as a reference: Carotid Artery intravasculaR Ultrasound Study (CARUS).Int J Cardiovasc Imaging. 2023 Oct;39(10):1909-1920. doi: 10.1007/s10554-023-02875-1. Epub 2023 Aug 21. Int J Cardiovasc Imaging. 2023. PMID: 37603155 Free PMC article.
-
The Effect of Lesion Length on Doppler Velocities Used Routinely to Determine Carotid Stenosis Cross-Sectional Severity.Diagnostics (Basel). 2025 May 15;15(10):1259. doi: 10.3390/diagnostics15101259. Diagnostics (Basel). 2025. PMID: 40428257 Free PMC article.
-
Inflammatory markers in patients with internal carotid artery stenosis.Arch Med Sci. 2013 Apr 20;9(2):254-60. doi: 10.5114/aoms.2013.34533. Epub 2013 Apr 9. Arch Med Sci. 2013. PMID: 23671435 Free PMC article.
-
Carotid artery Doppler ultrasonography in patients with chronic kidney disease.Med Sci Monit. 2014 Jan 7;20:11-7. doi: 10.12659/MSM.889857. Med Sci Monit. 2014. PMID: 24394695 Free PMC article.
-
Intravascular ultrasonography assisted carotid artery stenting for treatment of carotid stenosis: Two case reports.World J Clin Cases. 2023 Oct 16;11(29):7127-7135. doi: 10.12998/wjcc.v11.i29.7127. World J Clin Cases. 2023. PMID: 37946762 Free PMC article.
References
-
- Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. - PubMed
-
- Mead GE, Murray H, Farrell A, et al. Pilot study of carotid surgery for acute stroke. Br J Surg. 1997;84:990–92. - PubMed
-
- White CJ, Beckman JA, Cambria RP, et al. Atherosclerotic peripheral vascular disease symposium II: Controversies in carotid artery revascularization. Circulation. 2008;118:2852–59. - PubMed
-
- Roffi M, Spence JD. Is there a role for revascularisation in asymptomatic carotid stenosis? A Pro – Con debate. Brit Med J. 2010;341:584–85. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

