G-protein-coupled receptor kinase interactor-1 (GIT1) is a new endothelial nitric-oxide synthase (eNOS) interactor with functional effects on vascular homeostasis
- PMID: 22294688
- PMCID: PMC3320980
- DOI: 10.1074/jbc.M111.320465
G-protein-coupled receptor kinase interactor-1 (GIT1) is a new endothelial nitric-oxide synthase (eNOS) interactor with functional effects on vascular homeostasis
Abstract
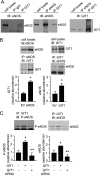
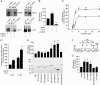
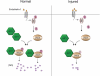
Endothelial cell nitric-oxide (NO) synthase (eNOS), the enzyme responsible for synthesis of NO in the vasculature, undergoes extensive post-translational modifications that modulate its activity. Here we have identified a novel eNOS interactor, G-protein-coupled receptor (GPCR) kinase interactor-1 (GIT1), which plays an unexpected role in GPCR stimulated NO signaling. GIT1 interacted with eNOS in the endothelial cell cytoplasm, and this robust association was associated with stimulatory eNOS phosphorylation (Ser(1177)), enzyme activation, and NO synthesis. GIT1 knockdown had the opposite effect. Additionally, GIT1 expression was reduced in sinusoidal endothelial cells after liver injury, consistent with previously described endothelial dysfunction in this disease. Re-expression of GIT1 after liver injury rescued the endothelial phenotype. These data emphasize the role of GPCR signaling partners in eNOS function and have fundamental implications for vascular disorders involving dysregulated eNOS.
Figures





Similar articles
-
Endothelial nitric-oxide synthase (eNOS) is activated through G-protein-coupled receptor kinase-interacting protein 1 (GIT1) tyrosine phosphorylation and Src protein.J Biol Chem. 2014 Jun 27;289(26):18163-74. doi: 10.1074/jbc.M113.521203. Epub 2014 Apr 24. J Biol Chem. 2014. PMID: 24764294 Free PMC article.
-
β-Arrestin2 is a critical component of the GPCR-eNOS signalosome.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11483-11492. doi: 10.1073/pnas.1922608117. Epub 2020 May 13. Proc Natl Acad Sci U S A. 2020. PMID: 32404425 Free PMC article.
-
β-PIX cooperates with GIT1 to regulate endothelial nitric oxide synthase in sinusoidal endothelial cells.Am J Physiol Gastrointest Liver Physiol. 2022 Nov 1;323(5):G511-G522. doi: 10.1152/ajpgi.00034.2022. Epub 2022 Aug 31. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 36044673 Free PMC article.
-
Life history of eNOS: partners and pathways.Cardiovasc Res. 2007 Jul 15;75(2):247-60. doi: 10.1016/j.cardiores.2007.03.023. Epub 2007 Apr 3. Cardiovasc Res. 2007. PMID: 17466957 Free PMC article. Review.
-
Endothelial NOS: perspective and recent developments.Br J Pharmacol. 2019 Jan;176(2):189-196. doi: 10.1111/bph.14522. Epub 2018 Dec 9. Br J Pharmacol. 2019. PMID: 30341769 Free PMC article. Review.
Cited by
-
The eNOS signalosome and its link to endothelial dysfunction.Pflugers Arch. 2016 Jul;468(7):1125-1137. doi: 10.1007/s00424-016-1839-0. Epub 2016 May 17. Pflugers Arch. 2016. PMID: 27184745 Review.
-
TSG (2,3,4' ,5-tetrahydroxystilbene 2-O-β-D-glucoside) suppresses induction of pro-inflammatory factors by attenuating the binding activity of nuclear factor-κB in microglia.J Neuroinflammation. 2013 Oct 21;10:129. doi: 10.1186/1742-2094-10-129. J Neuroinflammation. 2013. PMID: 24144353 Free PMC article.
-
Endothelial nitric-oxide synthase (eNOS) is activated through G-protein-coupled receptor kinase-interacting protein 1 (GIT1) tyrosine phosphorylation and Src protein.J Biol Chem. 2014 Jun 27;289(26):18163-74. doi: 10.1074/jbc.M113.521203. Epub 2014 Apr 24. J Biol Chem. 2014. PMID: 24764294 Free PMC article.
-
High-throughput deep screening and identification of four peripheral leucocyte microRNAs as novel potential combination biomarkers for preeclampsia.J Perinatol. 2016 Apr;36(4):263-7. doi: 10.1038/jp.2015.192. Epub 2015 Dec 17. J Perinatol. 2016. PMID: 26675000 Free PMC article.
-
Subcellular Localization Guides eNOS Function.Int J Mol Sci. 2024 Dec 13;25(24):13402. doi: 10.3390/ijms252413402. Int J Mol Sci. 2024. PMID: 39769167 Free PMC article. Review.
References
-
- Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. (1999) Activation of nitric-oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 - PubMed
-
- Busconi L., Michel T. (1993) Endothelial nitric-oxide synthase. N-terminal myristoylation determines subcellular localization. J. Biol. Chem. 268, 8410–8413 - PubMed
-
- García-Cardeña G., Fan R., Stern D. F., Liu J., Sessa W. C. (1996) Endothelial nitric-oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J. Biol. Chem. 271, 27237–27240 - PubMed
-
- Cao S., Yao J., McCabe T. J., Yao Q., Katusic Z. S., Sessa W. C., Shah V. (2001) Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J. Biol. Chem. 276, 14249–14256 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

