Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9
- PMID: 22294692
- PMCID: PMC3322827
- DOI: 10.1074/jbc.M111.319764
Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9
Abstract
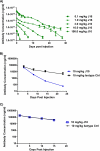
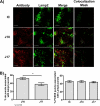
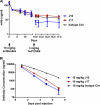
Target-mediated clearance and high antigen load can hamper the efficacy and dosage of many antibodies. We show for the first time that the mouse, cynomolgus, and human cross-reactive, antagonistic anti-proprotein convertase substilisin kexin type 9 (PCSK9) antibodies J10 and the affinity-matured and humanized J16 exhibit target-mediated clearance, resulting in dose-dependent pharmacokinetic profiles. These antibodies prevent the degradation of low density lipoprotein receptor, thus lowering serum levels of LDL-cholesterol and potently reducing serum cholesterol in mice, and selectively reduce LDL-cholesterol in cynomolgus monkeys. In order to increase the pharmacokinetic and efficacy of this promising therapeutic for hypercholesterolemia, we engineered pH-sensitive binding to mouse, cynomolgus, and human PCSK9 into J16, resulting in J17. This antibody shows prolonged half-life and increased duration of cholesterol lowering in two species in vivo by binding to endogenous PCSK9 in mice and cynomolgus monkeys, respectively. The proposed mechanism of this pH-sensitive antibody is that it binds with high affinity to PCSK9 in the plasma at pH 7.4, whereas the antibody-antigen complex dissociates at the endosomal pH of 5.5-6.0 in order to escape from target-mediated degradation. Additionally, this enables the antibody to bind to another PCSK9 and therefore increase the antigen-binding cycles. Furthermore, we show that this effect is dependent on the neonatal Fc receptor, which rescues the dissociated antibody in the endosome from degradation. Engineered pH-sensitive antibodies may enable less frequent or lower dosing of antibodies hampered by target-mediated clearance and high antigen load.
Figures

Similar articles
-
Anti-PCSK9 antibody pharmacokinetics and low-density lipoprotein-cholesterol pharmacodynamics in nonhuman primates are antigen affinity-dependent and exhibit limited sensitivity to neonatal Fc receptor-binding enhancement.J Pharmacol Exp Ther. 2015 Apr;353(1):119-31. doi: 10.1124/jpet.114.221242. Epub 2015 Feb 4. J Pharmacol Exp Ther. 2015. PMID: 25653417
-
Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates.J Pharmacol Exp Ther. 2012 Feb;340(2):228-36. doi: 10.1124/jpet.111.187419. Epub 2011 Oct 21. J Pharmacol Exp Ther. 2012. PMID: 22019884
-
Proteolytic cleavage of antigen extends the durability of an anti-PCSK9 monoclonal antibody.J Lipid Res. 2015 Nov;56(11):2124-32. doi: 10.1194/jlr.M061903. Epub 2015 Sep 20. J Lipid Res. 2015. PMID: 26392590 Free PMC article.
-
Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibition and the Future of Lipid Lowering Therapy.Prog Cardiovasc Dis. 2015 Jul-Aug;58(1):19-31. doi: 10.1016/j.pcad.2015.04.004. Epub 2015 May 1. Prog Cardiovasc Dis. 2015. PMID: 25936907 Review.
-
Biologics for the treatment of dyslipidemias: a look beyond conventional therapy.Ann Pharmacother. 2014 Feb;48(2):238-49. doi: 10.1177/1060028013511425. Epub 2013 Nov 6. Ann Pharmacother. 2014. PMID: 24259646 Review.
Cited by
-
Design of pH sensitive binding proteins from the hyperthermophilic Sso7d scaffold.PLoS One. 2012;7(11):e48928. doi: 10.1371/journal.pone.0048928. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23145025 Free PMC article.
-
The biology of PCSK9 from the endoplasmic reticulum to lysosomes: new and emerging therapeutics to control low-density lipoprotein cholesterol.Drug Des Devel Ther. 2013 Oct 4;7:1135-48. doi: 10.2147/DDDT.S36984. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 24115837 Free PMC article. Review.
-
Investigations of Influence of Antibody Binding Kinetics on Tumor Distribution and Anti-Tumor Efficacy.AAPS J. 2025 May 9;27(4):91. doi: 10.1208/s12248-025-01076-z. AAPS J. 2025. PMID: 40341444
-
Application of knockout mouse models to investigate the influence of FcγR on the pharmacokinetics and anti-platelet effects of MWReg30, a monoclonal anti-GPIIb antibody.Int J Pharm. 2013 Feb 28;444(1-2):185-92. doi: 10.1016/j.ijpharm.2013.01.001. Epub 2013 Jan 28. Int J Pharm. 2013. PMID: 23370434 Free PMC article.
-
High-Throughput Screening for Internalizing Antibodies by Homogeneous Fluorescence Imaging of a pH-Activated Probe.J Biomol Screen. 2016 Jan;21(1):12-23. doi: 10.1177/1087057115613270. Epub 2015 Oct 30. J Biomol Screen. 2016. PMID: 26518032 Free PMC article.
References
-
- Nelson A. L., Dhimolea E., Reichert J. M. (2010) Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug. Discov. 9, 767–774 - PubMed
-
- Yeung Y. A., Leabman M. K., Marvin J. S., Qiu J., Adams C. W., Lien S., Starovasnik M. A., Lowman H. B. (2009) Engineering human IgG1 affinity to human neonatal Fc receptor. Impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 182, 7663–7671 - PubMed
-
- Lambert G., Charlton F., Rye K. A., Piper D. E. (2009) Molecular basis of PCSK9 function. Atherosclerosis 203, 1–7 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

