Lipid polarity is maintained in absence of tight junctions
- PMID: 22294698
- PMCID: PMC3308754
- DOI: 10.1074/jbc.M111.327064
Lipid polarity is maintained in absence of tight junctions
Abstract
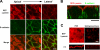
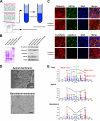
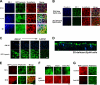
The role of tight junctions (TJs) in the establishment and maintenance of lipid polarity in epithelial cells has long been a subject of controversy. We have addressed this issue using lysenin, a toxin derived from earthworms, and an influenza virus labeled with a fluorescent lipid, octadecylrhodamine B (R18). When epithelial cells are stained with lysenin, lysenin selectively binds to their apical membranes. Using an artificial liposome, we demonstrated that lysenin recognizes the membrane domains where sphingomyelins are clustered. Interestingly, lysenin selectively stained the apical membranes of epithelial cells depleted of zonula occludens proteins (ZO-deficient cells), which completely lack TJs. Furthermore, the fluorescent lipid inserted into the apical membrane by fusion with the influenza virus did not diffuse to the lateral membrane in ZO-deficient epithelial cells. This study revealed that sphingomyelin-cluster formation occurs only in the apical membrane and that lipid polarity is maintained even in the absence of TJs.
Figures


References
-
- Gassama-Diagne A., Yu W., ter Beest M., Martin-Belmonte F., Kierbel A., Engel J., Mostov K. (2006) Phosphatidylinositol 3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat. Cell Biol. 8, 963–970 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

