Stress sensors and signal transducers in cyanobacteria
- PMID: 22294932
- PMCID: PMC3264485
- DOI: 10.3390/s100302386
Stress sensors and signal transducers in cyanobacteria
Abstract
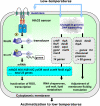
In living cells, the perception of environmental stress and the subsequent transduction of stress signals are primary events in the acclimation to changes in the environment. Some molecular sensors and transducers of environmental stress cannot be identified by traditional and conventional methods. Based on genomic information, a systematic approach has been applied to the solution of this problem in cyanobacteria, involving mutagenesis of potential sensors and signal transducers in combination with DNA microarray analyses for the genome-wide expression of genes. Forty-five genes for the histidine kinases (Hiks), 12 genes for serine-threonine protein kinases (Spks), 42 genes for response regulators (Rres), seven genes for RNA polymerase sigma factors, and nearly 70 genes for transcription factors have been successfully inactivated by targeted mutagenesis in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Screening of mutant libraries by genome-wide DNA microarray analysis under various stress and non-stress conditions has allowed identification of proteins that perceive and transduce signals of environmental stress. Here we summarize recent progress in the identification of sensory and regulatory systems, including Hiks, Rres, Spks, sigma factors, transcription factors, and the role of genomic DNA supercoiling in the regulation of the responses of cyanobacterial cells to various types of stress.
Keywords: histidine kinase; response regulator; sensor; serine-threonine kinase; stress; supercoiling; transducer.
Figures







References
-
- Glatz A., Vass I., Los D.A., Vigh L. The Synechocystis model of stress: from molecular chaperones to membranes. Plant. Physiol. Biochem. 1999;37:1–12.
-
- Los D.A., Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta. 2004;1666:142–157. - PubMed
-
- Williams J.G.K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis PCC6803. Method. Enzymol. 1988;167:766–778.
-
- Haselkorn R. Genetic systems in cyanobacteria. Method. Enzymol. 1991;204:418–430. - PubMed
-
- Elhai J., Wolk C.P. Conjugal transfer of DNA to cyanobacteria. Method. Enzymol. 1988;167:747–754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

