Highly accurate diagnosis of pleural tuberculosis by immunological analysis of the pleural effusion
- PMID: 22295081
- PMCID: PMC3266242
- DOI: 10.1371/journal.pone.0030324
Highly accurate diagnosis of pleural tuberculosis by immunological analysis of the pleural effusion
Abstract
Pleural TB is notoriously difficult to diagnose due to its paucibacillary nature yet it is the most common cause of pleural effusions in TB endemic countries such as The Gambia. We identified both cellular and soluble biomarkers in the pleural fluid that allowed highly accurate diagnosis of pleural TB compared to peripheral blood markers. Multi-plex cytokine analysis on unstimulated pleural fluid showed that IP-10 resulted in a positive likelihood ratio (LR) of 9.6 versus 2.8 for IFN-γ; a combination of IP-10, IL-6 and IL-10 resulted in an AUC of 0.96 and positive LR of 10. A striking finding was the significantly higher proportion of PPD-specific IFN-γ+TNF-α+ cell population (PPD-IGTA) in the pleural fluid compared to peripheral blood of TB subjects. Presence of this pleural PPD-IGTA population resulted in 95% correct classification of pleural TB disease with a sensitivity of 95% and specificity of 100%. These data suggest that analysis of the site of infection provides superior diagnostic accuracy compared to peripheral blood for pleural TB, likely due to the sequestration of effector cells at this acute stage of disease.
Conflict of interest statement
Figures
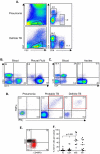


References
-
- WHO 2010–2011 TB Factsheet. Available at: www.who.int/tb/publications/2010/factsheet_tb_2010_rev21feb11.pdf/ Accessed 11th April, 2011.
-
- Porcel JM. Pearls and myths in pleural fluid analysis. Respirology. 2011;16:44–52. - PubMed
-
- Krenke R, Korczynski P. Use of pleural fluid levels of adenosine deaminase and interferon gamma in the diagnosis of tuberculous pleuritis. Curr Opin Pulm Med. 2010;16:367–375. - PubMed
-
- Hodsdon WS, Luzze H, Hurst TJ, Quigley MA, Kyosiimire J, et al. HIV-1-related pleural tuberculosis: elevated production of IFN-gamma, but failure of immunity to Mycobacterium tuberculosis. AIDS. 2001;15:467–475. - PubMed
-
- Hirsch CS, Toossi Z, Johnson JL, Luzze H, Ntambi L, et al. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis-infection in human tuberculosis. J Infect Dis. 2001;183:79–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

