LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease
- PMID: 22295101
- PMCID: PMC3266276
- DOI: 10.1371/journal.pone.0030668
LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease
Abstract
Background & aims: Non-alcoholic steatohepatitis (NASH) involves steatosis combined with inflammation, which can progress into fibrosis and cirrhosis. Exploring the molecular mechanisms of NASH is highly dependent on the availability of animal models. Currently, the most commonly used animal models for NASH imitate particularly late stages of human disease. Thus, there is a need for an animal model that can be used for investigating the factors that potentiate the inflammatory response within NASH. We have previously shown that 7-day high-fat-high-cholesterol (HFC) feeding induces steatosis and inflammation in both APOE2ki and Ldlr(-/-) mice. However, it is not known whether the early inflammatory response observed in these mice will sustain over time and lead to liver damage. We hypothesized that the inflammatory response in both models is sufficient to induce liver damage over time.
Methods: APOE2ki and Ldlr(-/-) mice were fed a chow or HFC diet for 3 months. C57Bl6/J mice were used as control.
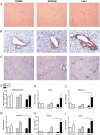
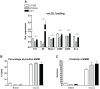
Results: Surprisingly, hepatic inflammation was abolished in APOE2ki mice, while it was sustained in Ldlr(-/-) mice. In addition, increased apoptosis and hepatic fibrosis was only demonstrated in Ldlr(-/-) mice. Finally, bone-marrow-derived-macrophages of Ldlr(-/-) mice showed an increased inflammatory response after oxidized LDL (oxLDL) loading compared to APOE2ki mice.
Conclusion: Ldlr(-/-) mice, but not APOE2ki mice, developed sustained hepatic inflammation and liver damage upon long term HFC feeding due to increased sensitivity for oxLDL uptake. Therefore, the Ldlr(-/-) mice are a promising physiological model particularly vulnerable for investigating the onset of hepatic inflammation in non-alcoholic steatohepatitis.
Conflict of interest statement
Figures


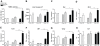

References
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. - PubMed
-
- Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. - PubMed
-
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

