Role of HER2-Targeted Agents in Adjuvant Treatment for Breast Cancer
- PMID: 22295205
- PMCID: PMC3263614
- DOI: 10.1155/2011/730360
Role of HER2-Targeted Agents in Adjuvant Treatment for Breast Cancer
Abstract
Approximately 20% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2) protein, mainly as a result of gene amplification. The receptor tyrosine kinase is believed to play a critical role in the pathogenesis and further proliferation of these tumors. The application of trastuzumab, a humanized monoclonal antibody against the extracellular domain of HER2 protein, to HER2-positive metastatic breast cancer has significantly improved treatment outcomes. Following this success, several phase III trials have evaluated the role of trastuzumab in the adjuvant setting, with the result that trastuzumab use is now the standard of care for most HER2-positive early breast cancer patients. In this paper, we review these pivotal phase III trials. We also discuss unresolved issues in adjuvant treatment with trastuzumab, including target patient population, sequential or concurrent use with chemotherapy or radiation, treatment duration, cardiotoxicity, and the possibility of eliminating chemotherapy. Following confirmation of its ability to partially overcome trastuzumab resistance, we also discuss the role of lapatinib in adjuvant use.
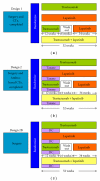
Figures

References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55(2):74–108. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. The New England Journal of Medicine. 2001;344(11):783–792. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

