Gold nanoparticles in chemical and biological sensing
- PMID: 22295941
- PMCID: PMC4102386
- DOI: 10.1021/cr2001178
Gold nanoparticles in chemical and biological sensing
Figures
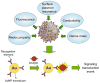
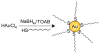
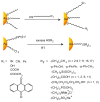
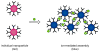



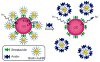

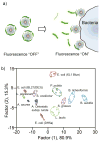
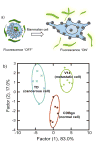

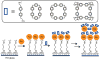

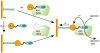
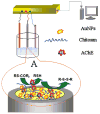

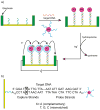
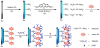
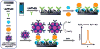

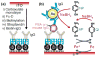
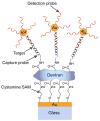


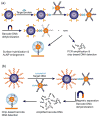
References
-
- Diamond D. Principles of Chemical and Biological Sensors. John Wiley & Sons, Inc; New York, NY: 1998.
-
- Sadik OA, Land WH, Wang J. Electroanalysis. 2003;15:1149–1159.
-
- Sapsford KE, Bradburne C, Detehanty JB, Medintz IL. Mater Today. 2008;11:38–49.
-
- Burnworth M, Rowan SJ, Weder C. Chem Eur J. 2007;13:7828–7836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

