Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection
- PMID: 22296706
- PMCID: PMC3395831
- DOI: 10.1186/1743-8977-9-3
Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection
Abstract
Background: Due to the rising use of nanomaterials (NMs), there is concern that NMs induce undesirable biological effects because of their unique physicochemical properties. Recently, we reported that amorphous silica nanoparticles (nSPs), which are one of the most widely used NMs, can penetrate the skin barrier and induce various biological effects, including an immune-modulating effect. Thus, it should be clarified whether nSPs can be a risk factor for the aggravation of skin immune diseases. Thus, in this study, we investigated the relationship between the size of SPs and adjuvant activity using a model for atopic dermatitis.
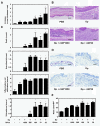
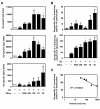
Results: We investigated the effects of nSPs on the AD induced by intradermaly injected-mite antigen Dermatophagoides pteronyssinus (Dp) in NC/Nga mice. Ear thickness measurements and histopathological analysis revealed that a combined injection of amorphous silica particles (SPs) and Dp induced aggravation of AD in an SP size-dependent manner compared to that of Dp alone. In particular, aggravation was observed remarkably in nSP-injected groups. Furthermore, these effects were correlated with the excessive induction of total IgE and a stronger systemic Th2 response. We demonstrated that these results are associated with the induction of IL-18 and thymic stromal lymphopoietin (TSLP) in the skin lesions.
Conclusions: A particle size reduction in silica particles enhanced IL-18 and TSLP production, which leads to systemic Th2 response and aggravation of AD-like skin lesions as induced by Dp antigen treatment. We believe that appropriate regulation of nanoparticle physicochemical properties, including sizes, is a critical determinant for the design of safer forms of NMs.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

