Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis
- PMID: 22296834
- PMCID: PMC3381520
- DOI: 10.1126/scisignal.2002446
Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis
Abstract
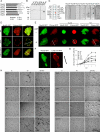
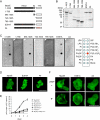
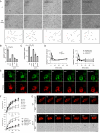
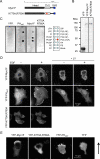
Class I myosins participate in various interactions between the cell membrane and the cytoskeleton. Several class I myosins preferentially bind to acidic phospholipids, such as phosphatidylserine and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], through a tail homology 1 (TH1) domain. Here, we show that the second messenger lipid phosphatidylinositol 3,4,5-trisphosphate (PIP3) binds to the TH1 domain of a subset of Dictyostelium class I myosins (ID, IE, and IF) and recruits them to the plasma membrane. The PIP3-regulated membrane recruitment of myosin I promoted chemotaxis and induced chemoattractant-stimulated actin polymerization. Similarly, PIP3 recruited human myosin IF to the plasma membrane upon chemotactic stimulation in a neutrophil cell line. These data suggest a mechanism through which the PIP3 signal is transmitted through myosin I to the actin cytoskeleton.
Figures
References
-
- Van Haastert PJ. Chemotaxis: insights from the extending pseudopod. J Cell Sci. 2010;123:3031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

