The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study
- PMID: 22297124
- PMCID: PMC3533918
- DOI: 10.1093/eurheartj/ehr504
The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study
Abstract
Aims: Patients with chronic heart failure (CHF) show impaired health-related quality of life (HRQoL), an important target for therapeutic intervention. Impaired iron homeostasis may be one mechanism underlying the poor physical condition of CHF patients. This detailed subanalysis of the previously published FAIR-HF study evaluated baseline HRQoL in iron-deficient patients with CHF and the effect of intravenous ferric carboxymaltose (FCM) on HRQoL.
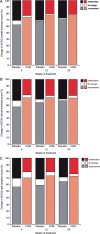
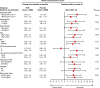
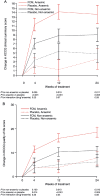
Methods and results: FAIR-HF randomized 459 patients with reduced left ventricular ejection fraction and iron deficiency, with or without anaemia, to FCM or placebo (2:1). Health-related quality of life was assessed at baseline and after 4, 12, and 24 weeks of therapy using the generic EQ-5D questionnaire and disease-specific Kansas City cardiomyopathy questionnaire (KCCQ). Baseline mean visual analogue scale (VAS) score was 54.3 ± 16.4 and KCCQ overall summary score was 52.4 ± 18.8. Ferric carboxymaltose significantly improved VAS and KCCQ (mean differences from baseline in KCCQ overall, clinical and total symptom scores, P< 0.001 vs. placebo) at all time points. At week 24, significant improvement vs. placebo was observed in four of the five EQ-5D dimensions: mobility (P= 0.004), self-care (P< 0.001), pain/discomfort (P= 0.006), anxiety/depression (P= 0.012), and usual activity (P= 0.035). Ferric carboxymaltose improved all KCCQ domain mean scores from Week 4 onward (P≤ 0.05), except for self-efficacy and social limitation. Effects were present in both anaemic and non-anaemic patients.
Conclusions: HRQoL is impaired in iron-deficient patients with CHF. Intravenous FCM significantly improved HRQoL after 4 weeks, and throughout the remaining study period. The positive effects of FCM were independent of anaemia status.
Figures

References
-
- Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. - PubMed
-
- Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002;23:1867–1876. - PubMed
-
- Witte KK, Clark AL. Why does chronic heart failure cause breathlessness and fatigue? Prog Cardiovasc Dis. 2007;49:366–384. - PubMed
-
- Gravely-Witte S, Jurgens CY, Tamim H, Grace SL. Length of delay in seeking medical care by patients with heart failure symptoms and the role of symptom-related factors: a narrative review. Eur J Heart Fail. 2010;12:1122–1129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

