Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial
- PMID: 22297150
- PMCID: PMC3343177
- DOI: 10.1016/j.biopsych.2011.12.010
Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial
Abstract
Background: Currently, no pharmacological treatments for bipolar depression exist that exert rapid (within hours) antidepressant or antisuicidal effects. We previously reported that intravenous administration of the N-methyl-D-aspartate antagonist ketamine produced rapid antidepressant effects in patients with treatment-resistant bipolar depression. The present study sought to replicate this finding in an independent sample.
Methods: In this double-blind, randomized, crossover, placebo-controlled study, 15 subjects with DSM-IV bipolar I or II depression maintained on therapeutic levels of lithium or valproate received a single intravenous infusion of either ketamine hydrochloride (.5 mg/kg) or placebo on 2 test days 2 weeks apart. The primary outcome measure was the Montgomery-Asberg Depression Rating Scale, which was used to rate overall depressive symptoms at baseline; at 40, 80, 110, and 230 minutes postinfusion; and on days 1, 2, 3, 7, 10, and 14 postinfusion.
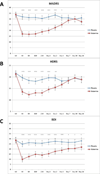
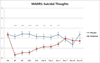
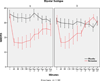
Results: Within 40 minutes, depressive symptoms, as well as suicidal ideation, significantly improved in subjects receiving ketamine compared with placebo (d = .89, 95% confidence interval = .61-1.16, and .98, 95% confidence interval = .64-1.33, respectively); this improvement remained significant through day 3. Seventy-nine percent of subjects responded to ketamine and 0% responded to placebo at some point during the trial. The most common side effect was dissociative symptoms, which occurred only at the 40-minute time point.
Conclusions: This study replicated our previous finding that patients with bipolar depression who received a single ketamine infusion experienced a rapid and robust antidepressant response. In addition, we found that ketamine rapidly improved suicidal ideation in these patients.
Trial registration: ClinicalTrials.gov NCT00088699.
Copyright © 2012 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All other authors report no biomedical financial interests or potential conflicts of interest.
Figures
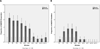
References
-
- Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68:1058–1064. - PubMed
-
- Post RM, Leverich GS, Altshuler LL, Frye MA, Suppes T, Keck PE, et al. Differential clinical characteristics, medication usage, and treatment response of bipolar disorder in the US versus The Netherlands and Germany. Int Clin Psychopharmacol. 2011;26:96–106. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

