Crystallization and preliminary X-ray diffraction analysis of human dipeptidyl peptidase 10 (DPPY), a component of voltage-gated potassium channels
- PMID: 22298003
- PMCID: PMC3274407
- DOI: 10.1107/S1744309111055230
Crystallization and preliminary X-ray diffraction analysis of human dipeptidyl peptidase 10 (DPPY), a component of voltage-gated potassium channels
Abstract
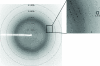
Dipeptidyl peptidase 10 (DPP10, DPPY) is an inactive peptidase associated with voltage-gated potassium channels, acting as a modulator of their electrophysiological properties, cell-surface expression and subcellular localization. Because potassium channels are important disease targets, biochemical and structural characterization of their interaction partners was sought. DPP10 was cloned and expressed using an insect-cell system and the protein was purified via His-tag affinity and size-exclusion chromatography. Crystals obtained by the sitting-drop method were orthorhombic, belonging to space group P2(1)2(1)2(1) with unit-cell parameters a = 80.91, b = 143.73, c = 176.25 Å. A single solution with two molecules in the asymmetric unit was found using the structure of DPP6 (also called DPPX; PDB entry 1xfd) as the search model in a molecular replacement protocol.
Figures
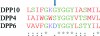

References
-
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
-
- Allen, M. et al. (2003). Nature Genet. 35, 258–263. - PubMed
-
- Chen, T., Shen, X.-F., Chegini, F., Gai, W.-P. & Abbott, C. A. (2008). Clin. Chem. Lab. Med. 46, A13.
-
- Djurovic, S., Gustafsson, O., Mattingsdal, M., Athanasiu, L., Bjella, T., Tesli, M., Agartz, I., Lorentzen, S., Melle, I., Morken, G. & Andreassen, O. A. (2010). J. Affect. Disord. 126, 312–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

