Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study
- PMID: 22298121
- PMCID: PMC3327857
- DOI: 10.1038/npp.2011.338
Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study
Abstract
The N-methyl-D-aspartate antagonist ketamine has rapid antidepressant effects in patients with treatment-resistant major depression (TRD); these effects have been reported to last for 1 week in some patients. However, the extent and duration of this antidepressant effect over longer periods has not been well characterized under controlled conditions. Riluzole, a glutamatergic modulator with antidepressant and synaptic plasticity-enhancing effects, could conceivably be used to promote the antidepressant effects of ketamine. This study sought to determine the extent and time course of antidepressant improvement to a single-ketamine infusion over 4 weeks, comparing the addition of riluzole vs placebo after the infusion. Forty-two subjects (18-65) with TRD and a Montgomery-Asberg Depression Rating Scale (MADRS) score of ≥ 22 received a single intravenous infusion of ketamine (0.5 mg/kg). Four to six hours post-infusion, subjects were randomized to double-blind treatment with either riluzole (100-200 mg/day; n=21) or placebo (n=21) for 4 weeks. Depressive symptoms were rated daily. A significant improvement (P<0.001) in MADRS scores from baseline was found. The effect size of improvement with ketamine was initially large and remained moderate throughout the 28-day trial. Overall, 27% of ketamine responders had not relapsed by 4 weeks following a single ketamine infusion. The average time to relapse was 13.2 days (SE=2.2). However, the difference between the riluzole and placebo treatment groups was not significant, suggesting that the combination of riluzole with ketamine treatment did not significantly alter the course of antidepressant response to ketamine alone.
Figures

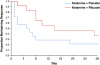
References
-
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. - PubMed
-
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. - PubMed
-
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

