Epoxides and soluble epoxide hydrolase in cardiovascular physiology
- PMID: 22298653
- PMCID: PMC3613253
- DOI: 10.1152/physrev.00021.2011
Epoxides and soluble epoxide hydrolase in cardiovascular physiology
Abstract
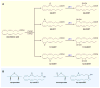
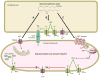
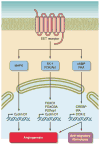
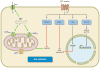
Epoxyeicosatrienoic acids (EETs) are arachidonic acid metabolites that importantly contribute to vascular and cardiac physiology. The contribution of EETs to vascular and cardiac function is further influenced by soluble epoxide hydrolase (sEH) that degrades EETs to diols. Vascular actions of EETs include dilation and angiogenesis. EETs also decrease inflammation and platelet aggregation and in general act to maintain vascular homeostasis. Myocyte contraction and increased coronary blood flow are the two primary EET actions in the heart. EET cell signaling mechanisms are tissue and organ specific and provide significant evidence for the existence of EET receptors. Additionally, pharmacological and genetic manipulations of EETs and sEH have demonstrated a contribution for this metabolic pathway to cardiovascular diseases. Given the impact of EETs to cardiovascular physiology, there is emerging evidence that development of EET-based therapeutics will be beneficial for cardiovascular diseases.
Figures
References
-
- Adeagbo ASO, Oyekan AO, Joshua IG, Falkner KC, Prough RA, Pierce WM. Epoxyeicosatrienoic acid release mediates nitric oxide-independent dilation of rat mesenteric vessels. In: Vanhoutte PM, editor. EDHF. London: Taylor & Francis; 2001. pp. 195–206.
-
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. - PubMed
-
- Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, Hurn PJ. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33:1677–1684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

