Regulation and function of the FGF23/klotho endocrine pathways
- PMID: 22298654
- PMCID: PMC3306265
- DOI: 10.1152/physrev.00002.2011
Regulation and function of the FGF23/klotho endocrine pathways
Abstract
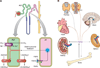
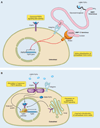
Calcium (Ca(2+)) and phosphate (PO(4)(3-)) homeostasis are coordinated by systemic and local factors that regulate intestinal absorption, influx and efflux from bone, and kidney excretion and reabsorption of these ions through a complex hormonal network. Traditionally, the parathyroid hormone (PTH)/vitamin D axis provided the conceptual framework to understand mineral metabolism. PTH secreted by the parathyroid gland in response to hypocalcemia functions to maintain serum Ca(2+) levels by increasing Ca(2+) reabsorption and 1,25-dihydroxyvitamin D [1,25(OH)(2)D] production by the kidney, enhancing Ca(2+) and PO(4)(3-) intestinal absorption and increasing Ca(2+) and PO(4)(3-) efflux from bone, while maintaining neutral phosphate balance through phosphaturic effects. FGF23 is a recently discovered hormone, predominately produced by osteoblasts/osteocytes, whose major functions are to inhibit renal tubular phosphate reabsorption and suppress circulating 1,25(OH)(2)D levels by decreasing Cyp27b1-mediated formation and stimulating Cyp24-mediated catabolism of 1,25(OH)(2)D. FGF23 participates in a new bone/kidney axis that protects the organism from excess vitamin D and coordinates renal PO(4)(3-) handling with bone mineralization/turnover. Abnormalities of FGF23 production underlie many inherited and acquired disorders of phosphate homeostasis. This review discusses the known and emerging functions of FGF23, its regulation in response to systemic and local signals, as well as the implications of FGF23 in different pathological and physiological contexts.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–1649. - PubMed
-
- ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. - PubMed
-
- Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. - PubMed
-
- Anamizu Y, Kawaguchi H, Seichi A, Yamaguchi S, Kawakami E, Kanda N, Matsubara S, Kuro-o M, Nabeshima Y, Nakamura K, Oyanagi K. Klotho insufficiency causes decrease of ribosomal RNA gene transcription activity, cytoplasmic RNA and rough ER in the spinal anterior horn cells. Acta Neuropathol. 2005;109:457–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

