Organ-specific sulfation patterns of heparan sulfate generated by extracellular sulfatases Sulf1 and Sulf2 in mice
- PMID: 22298771
- PMCID: PMC3308746
- DOI: 10.1074/jbc.M111.290262
Organ-specific sulfation patterns of heparan sulfate generated by extracellular sulfatases Sulf1 and Sulf2 in mice
Abstract
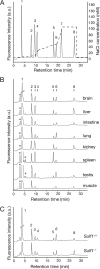
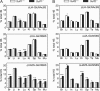
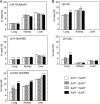
Heparan sulfate endosulfatases Sulf1 and Sulf2 hydrolyze 6-O-sulfate in heparan sulfate, thereby regulating cellular signaling. Previous studies have revealed that Sulfs act predominantly on UA2S-GlcNS6S disaccharides and weakly on UA-GlcNS6S disaccharides. However, the specificity of Sulfs and their role in sulfation patterning of heparan sulfate in vivo remained unknown. Here, we performed disaccharide analysis of heparan sulfate in Sulf1 and Sulf2 knock-out mice. Significant increases in ΔUA2S-GlcNS6S were observed in the brain, small intestine, lung, spleen, testis, and skeletal muscle of adult Sulf1(-/-) mice and in the brain, liver, kidney, spleen, and testis of adult Sulf2(-/-) mice. In addition, increases in ΔUA-GlcNS6S were seen in the Sulf1(-/-) lung and small intestine. In contrast, the disaccharide compositions of chondroitin sulfate were not primarily altered, indicating specificity of Sulfs for heparan sulfate. For Sulf1, but not for Sulf2, mRNA expression levels in eight organs of wild-type mice were highly correlated with increases in ΔUA2S-GlcNS6S in the corresponding organs of knock-out mice. Moreover, overall changes in heparan sulfate compositions were greater in Sulf1(-/-) mice than in Sulf2(-/-) mice despite lower levels of Sulf1 mRNA expression, suggesting predominant roles of Sulf1 in heparan sulfate desulfation and distinct regulation of Sulf activities in vivo. Sulf1 and Sulf2 mRNAs were differentially expressed in restricted types of cells in organs, and consequently, the sulfation patterns of heparan sulfate were locally and distinctly altered in Sulf1 and Sulf2 knock-out mice. These findings indicate that Sulf1 and Sulf2 differentially contribute to the generation of organ-specific sulfation patterns of heparan sulfate.
Figures




References
-
- Lindahl U., Kusche-Gullberg M., Kjellén L. (1998) Regulated diversity of heparan sulfate. J. Biol. Chem. 273, 24979–24982 - PubMed
-
- Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 - PubMed
-
- Turnbull J., Powell A., Guimond S. (2001) Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11, 75–82 - PubMed
-
- Esko J. D., Selleck S. B. (2002) Order out of chaos. Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

