TGF-β and BMP signaling in osteoblast differentiation and bone formation
- PMID: 22298955
- PMCID: PMC3269610
- DOI: 10.7150/ijbs.2929
TGF-β and BMP signaling in osteoblast differentiation and bone formation
Abstract
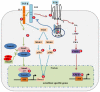
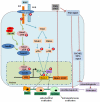
Transforming growth factor-beta (TGF-β)/bone morphogenic protein (BMP) signaling is involved in a vast majority of cellular processes and is fundamentally important throughout life. TGF-β/BMPs have widely recognized roles in bone formation during mammalian development and exhibit versatile regulatory functions in the body. Signaling transduction by TGF-β/BMPs is specifically through both canonical Smad-dependent pathways (TGF-β/BMP ligands, receptors and Smads) and non-canonical Smad-independent signaling pathway (e.g. p38 mitogen-activated protein kinase pathway, MAPK). Following TGF-β/BMP induction, both the Smad and p38 MAPK pathways converge at the Runx2 gene to control mesenchymal precursor cell differentiation. The coordinated activity of Runx2 and TGF-β/BMP-activated Smads is critical for formation of the skeleton. Recent advances in molecular and genetic studies using gene targeting in mice enable a better understanding of TGF-β/BMP signaling in bone and in the signaling networks underlying osteoblast differentiation and bone formation. This review summarizes the recent advances in our understanding of TGF-β/BMP signaling in bone from studies of genetic mouse models and human diseases caused by the disruption of TGF-β/BMP signaling. This review also highlights the different modes of cross-talk between TGF-β/BMP signaling and the signaling pathways of MAPK, Wnt, Hedgehog, Notch, and FGF in osteoblast differentiation and bone formation.
Keywords: BMP signaling; Bone; Osteoblasts; Runx2; Smad; TGF signaling.
Conflict of interest statement
Conflict of Interests: The authors have declared that no conflict of interest exists.
Figures
References
-
- Wagner DO, Sieber C, Bhushan R, Borgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3:mr1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

