Systemic inflammatory profile and response to anti-tumor necrosis factor therapy in chronic obstructive pulmonary disease
- PMID: 22300528
- PMCID: PMC3287122
- DOI: 10.1186/1465-9921-13-12
Systemic inflammatory profile and response to anti-tumor necrosis factor therapy in chronic obstructive pulmonary disease
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterized by progressive worsening of airflow limitation associated with abnormally inflamed airways in older smokers. Despite correlative evidence for a role for tumor necrosis factor-alpha in the pathogenesis of COPD, the anti-tumor necrosis factor-alpha, infliximab did not show clinical efficacy in a double-blind, placebo-controlled, phase II clinical trial. This study sought to evaluate the systemic inflammatory profile associated with COPD and to assess the impact of tumor necrosis factor neutralization on systemic inflammation.
Methods: Serum samples (n = 234) from the phase II trial were collected at baseline and after 24 weeks of placebo or infliximab. Additionally, baseline serum samples were obtained from an independent COPD cohort (n = 160) and 2 healthy control cohorts (n = 50; n = 109). Serum concentrations of a broad panel of inflammation-associated analytes were measured using a 92-analyte multiplex assay.
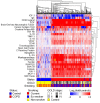
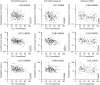
Results: Twenty-five proteins were significantly elevated and 2 were decreased in COPD, including highly elevated CD40 ligand, brain-derived neurotrophic factor, epidermal growth factor, acute-phase proteins, and neutrophil-associated proteins. This profile was largely independent of smoking status, age, and clinical phenotype. The majority of these associations of serum analytes with COPD are novel findings. Increased serum creatine kinase-muscle/brain and myoglobin correlated modestly with decreased forced expiratory volume at 1 second, suggesting cardiac involvement. Infliximab did not affect this systemic inflammatory profile.
Conclusions: A robust systemic inflammatory profile was associated with COPD. This profile was generally independent of disease severity. Because anti-tumor necrosis factor-alpha did not influence systemic inflammation, how to control the underlying pathology beyond symptom suppression remains unclear.
Trial registration: ClinicalTrials.gov NCT00056264.
Figures


Similar articles
-
Effect of infliximab on local and systemic inflammation in chronic obstructive pulmonary disease: a pilot study.Respiration. 2008;76(3):275-82. doi: 10.1159/000117386. Epub 2008 Feb 15. Respiration. 2008. PMID: 18277064 Clinical Trial.
-
Inflammatory profile and response to anti-tumor necrosis factor therapy in patients with chronic pulmonary sarcoidosis.Clin Vaccine Immunol. 2011 Jun;18(6):931-9. doi: 10.1128/CVI.00337-10. Epub 2011 Apr 20. Clin Vaccine Immunol. 2011. PMID: 21508170 Free PMC article. Clinical Trial.
-
The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease.Am J Respir Crit Care Med. 2007 May 1;175(9):926-34. doi: 10.1164/rccm.200607-995OC. Epub 2007 Feb 8. Am J Respir Crit Care Med. 2007. PMID: 17290043 Clinical Trial.
-
Anti-TNF-alpha therapies in chronic obstructive pulmonary diseases.Expert Opin Investig Drugs. 2008 Aug;17(8):1203-11. doi: 10.1517/13543784.17.8.1203. Expert Opin Investig Drugs. 2008. PMID: 18616416 Review.
-
TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water.Pulm Pharmacol Ther. 2010 Apr;23(2):121-8. doi: 10.1016/j.pupt.2009.10.007. Epub 2009 Oct 22. Pulm Pharmacol Ther. 2010. PMID: 19853667 Review.
Cited by
-
Development and validation of a prospective study to predict the risk of readmission within 365 days of respiratory failure: based on a random survival forest algorithm combined with COX regression modeling.BMC Pulm Med. 2024 Feb 14;24(1):82. doi: 10.1186/s12890-024-02862-9. BMC Pulm Med. 2024. PMID: 38355552 Free PMC article.
-
Insulin-Like Growth Factor-1 Signaling in Lung Development and Inflammatory Lung Diseases.Biomed Res Int. 2018 Jun 19;2018:6057589. doi: 10.1155/2018/6057589. eCollection 2018. Biomed Res Int. 2018. PMID: 30018981 Free PMC article. Review.
-
Transcriptome-Guided Drug Repositioning.Pharmaceutics. 2019 Dec 12;11(12):677. doi: 10.3390/pharmaceutics11120677. Pharmaceutics. 2019. PMID: 31842375 Free PMC article.
-
The Molecular Blueprint for Chronic Obstructive Pulmonary Disease (COPD): A New Paradigm for Diagnosis and Therapeutics.Oxid Med Cell Longev. 2023 Dec 21;2023:2297559. doi: 10.1155/2023/2297559. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 38155869 Free PMC article. Review.
-
Airway Disease in Rheumatoid Arthritis.Ann Am Thorac Soc. 2022 Mar;19(3):343-352. doi: 10.1513/AnnalsATS.202107-876CME. Ann Am Thorac Soc. 2022. PMID: 34929135 Free PMC article. Review.
References
-
- Sakao S, Tatsumi K, Igari H, Shino Y, Shirasawa H, Kuriyama T. Association of tumor necrosis factor alpha gene promoter polymorphism with the presence of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(2):420–422. - PubMed
-
- Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, Dales RE. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(2):349–355. - PubMed
-
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1453–1455. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

