Copy number variation of individual cattle genomes using next-generation sequencing
- PMID: 22300768
- PMCID: PMC3317159
- DOI: 10.1101/gr.133967.111
Copy number variation of individual cattle genomes using next-generation sequencing
Abstract
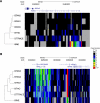
Copy number variations (CNVs) affect a wide range of phenotypic traits; however, CNVs in or near segmental duplication regions are often intractable. Using a read depth approach based on next-generation sequencing, we examined genome-wide copy number differences among five taurine (three Angus, one Holstein, and one Hereford) and one indicine (Nelore) cattle. Within mapped chromosomal sequence, we identified 1265 CNV regions comprising ~55.6-Mbp sequence--476 of which (~38%) have not previously been reported. We validated this sequence-based CNV call set with array comparative genomic hybridization (aCGH), quantitative PCR (qPCR), and fluorescent in situ hybridization (FISH), achieving a validation rate of 82% and a false positive rate of 8%. We further estimated absolute copy numbers for genomic segments and annotated genes in each individual. Surveys of the top 25 most variable genes revealed that the Nelore individual had the lowest copy numbers in 13 cases (~52%, χ(2) test; P-value <0.05). In contrast, genes related to pathogen- and parasite-resistance, such as CATHL4 and ULBP17, were highly duplicated in the Nelore individual relative to the taurine cattle, while genes involved in lipid transport and metabolism, including APOL3 and FABP2, were highly duplicated in the beef breeds. These CNV regions also harbor genes like BPIFA2A (BSP30A) and WC1, suggesting that some CNVs may be associated with breed-specific differences in adaptation, health, and production traits. By providing the first individualized cattle CNV and segmental duplication maps and genome-wide gene copy number estimates, we enable future CNV studies into highly duplicated regions in the cattle genome.
Figures



References
-
- Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, et al. 2006. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439: 851–855 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
