Performance of tuberculosis drug susceptibility testing in U.S. laboratories from 1994 to 2008
- PMID: 22301024
- PMCID: PMC3318540
- DOI: 10.1128/JCM.06479-11
Performance of tuberculosis drug susceptibility testing in U.S. laboratories from 1994 to 2008
Abstract
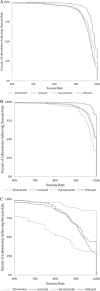
We present a statistical summary of results from the Model Performance Evaluation Program (MPEP) for Mycobacterium tuberculosis Drug Susceptibility Testing, 1994 to 2008, implemented by the U.S. Centers for Disease Control and Prevention (CDC). During that period, a total of 57,733 test results for culture isolates were reported by 216 participating laboratories for the first-line antituberculosis drugs used in the United States-isoniazid (INH), rifampin (RMP), ethambutol (EMB), and pyrazinamide (PZA). Using Clinical Laboratory and Standards Institute (CLSI)-recommended concentrations for one or more of three methods, agar proportion (AP), BACTEC460 (Bactec), and MGIT-960 (MGIT), yielded overall agreement of 97.0% for first-line drugs. For susceptible strains, agreement was 98.4%; for resistant strains, agreement was 91.0%, with significantly lower accuracy (chi-square test, P < 0.0001). For resistant strains, overall agreement by methods was 91.3% for AP, 93.0% for Bactec, and 82.6% for MGIT and by drugs was 92.2% for INH, 91.5% for RMP, 79.0% for EMB, and 97.5% for PZA. For some strains, performance by method varied significantly. Use of duplicate strains in the same shipment and repeat strains over time revealed consistent performance even for strains with higher levels of interlaboratory discordance. No overall differences in performance between laboratories were observed based on volume of testing or type of facility (e.g., health department, hospital, independent). By all methods, decreased performance was observed for strains with low-level INH resistance, RMP resistance, and EMB-resistant strains. These results demonstrate a high level of performance in detection of drug-resistant M. tuberculosis in U.S. laboratories.
Figures

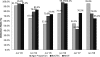
Similar articles
-
Comparison of line probe assay to BACTEC MGIT 960 system for susceptibility testing of first and second-line anti-tuberculosis drugs in a referral laboratory in South Africa.BMC Infect Dis. 2017 Dec 28;17(1):795. doi: 10.1186/s12879-017-2898-3. BMC Infect Dis. 2017. PMID: 29282012 Free PMC article.
-
Evaluation of the fully automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide, streptomycin, isoniazid, rifampin, and ethambutol and comparison with the radiometric BACTEC 460TB method.J Clin Microbiol. 2004 Mar;42(3):1109-14. doi: 10.1128/JCM.42.3.1109-1114.2004. J Clin Microbiol. 2004. PMID: 15004061 Free PMC article.
-
Evaluation of BACTEC Mycobacteria Growth Indicator Tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis.J Clin Microbiol. 2001 Dec;39(12):4440-4. doi: 10.1128/JCM.39.12.4440-4444.2001. J Clin Microbiol. 2001. PMID: 11724858 Free PMC article.
-
Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Reference Laboratory Network: five rounds of proficiency testing, 1994-1998.Int J Tuberc Lung Dis. 2002 Sep;6(9):748-56. Int J Tuberc Lung Dis. 2002. PMID: 12234129
-
Multicenter evaluation of ethambutol susceptibility testing of mycobacterium tuberculosis by agar proportion and radiometric methods.J Clin Microbiol. 2002 Nov;40(11):3976-9. doi: 10.1128/JCM.40.11.3976-3979.2002. J Clin Microbiol. 2002. PMID: 12409361 Free PMC article.
Cited by
-
Utilization of whole genome sequencing for resolution of discrepant Mycobacterium tuberculosis drug susceptibility results: A case report.IDCases. 2021 Oct 8;26:e01308. doi: 10.1016/j.idcr.2021.e01308. eCollection 2021. IDCases. 2021. PMID: 34745885 Free PMC article.
-
Epidemiology of pyrazinamide-resistant tuberculosis in the United States, 1999-2009.Clin Infect Dis. 2013 Oct;57(8):1081-93. doi: 10.1093/cid/cit452. Epub 2013 Jul 9. Clin Infect Dis. 2013. PMID: 23840002 Free PMC article.
-
Discordance across Phenotypic and Molecular Methods for Drug Susceptibility Testing of Drug-Resistant Mycobacterium tuberculosis Isolates in a Low TB Incidence Country.PLoS One. 2016 Apr 20;11(4):e0153563. doi: 10.1371/journal.pone.0153563. eCollection 2016. PLoS One. 2016. PMID: 27096759 Free PMC article.
-
Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: investigation of cases with discrepant susceptibility results.PLoS One. 2014 Mar 5;9(3):e90569. doi: 10.1371/journal.pone.0090569. eCollection 2014. PLoS One. 2014. PMID: 24599230 Free PMC article.
-
Rifampin-resistant Tuberculosis in the United States, 1998-2014.Clin Infect Dis. 2020 Apr 10;70(8):1596-1605. doi: 10.1093/cid/ciz491. Clin Infect Dis. 2020. PMID: 31233131 Free PMC article.
References
-
- Böttger E. 2011. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin. Microbiol. Infect. 17:1128–1134 - PubMed
-
- Centers for Disease Control and Prevention 1995. Laboratory practices for diagnosis of tuberculosis—United States, 1994. Morb. Mortal. Wkly. Rep. 44:587–590 - PubMed
-
- Centers for Disease Control and Prevention 1992. National action plan to combat multidrug-resistant tuberculosis. Morb. Mortal. Wkly. Rep. Recom. Rep. 41:1–48 - PubMed
-
- Centers for Disease Control and Prevention 2010. Reported tuberculosis in the United States, 2009. U.S. Department of Health and Human Services, Atlanta, GA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

