Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-I and MDA-5
- PMID: 22301138
- PMCID: PMC3302539
- DOI: 10.1128/JVI.06713-11
Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-I and MDA-5
Abstract
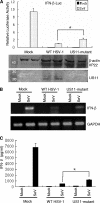
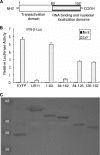
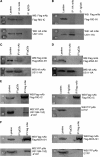
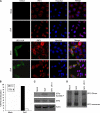
The interferon (IFN)-mediated antiviral response is a major defense of the host immune system. In order to complete their life cycle, viruses must modulate host IFN-mediated immune responses. Herpes simplex virus 1 (HSV-1) is a large DNA virus containing more than 80 genes, many of which encode proteins that are involved in virus-host interactions and show immune modulatory capabilities. In this study, we demonstrate that the US11 protein, an RNA binding tegument protein of HSV-1, is a novel antagonist of the beta IFN (IFN-β) pathway. US11 significantly inhibited Sendai virus (SeV)-induced IFN-β production, and its double-stranded RNA (dsRNA) binding domain was indispensable for this inhibition activity. Additionally, wild-type HSV-1 coinfection showed stronger inhibition than US11 mutant HSV-1 in SeV-induced IFN-β production. Coimmunoprecipitation analysis demonstrated that the US11 protein in HSV-1-infected cells interacts with endogenous RIG-I and MDA-5 through its C-terminal RNA-binding domain, which was RNA independent. Expression of US11 in both transfected and HSV-1-infected cells interferes with the interaction between MAVS and RIG-I or MDA-5. Finally, US11 dampens SeV-mediated IRF3 activation. Taken together, the combined data indicate that HSV-1 US11 binds to RIG-I and MDA-5 and inhibits their downstream signaling pathway, preventing the production of IFN-β, which may contribute to the pathogenesis of HSV-1 infection.
Figures



Similar articles
-
Suppression of PACT-induced type I interferon production by herpes simplex virus 1 Us11 protein.J Virol. 2013 Dec;87(24):13141-9. doi: 10.1128/JVI.02564-13. Epub 2013 Sep 25. J Virol. 2013. PMID: 24067967 Free PMC article.
-
Herpes Simplex Virus 1 Inhibits TANK-Binding Kinase 1 through Formation of the Us11-Hsp90 Complex.J Virol. 2018 Jun 29;92(14):e00402-18. doi: 10.1128/JVI.00402-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743370 Free PMC article.
-
The Molecular Mechanism of Herpes Simplex Virus 1 UL31 in Antagonizing the Activity of IFN-β.Microbiol Spectr. 2022 Feb 23;10(1):e0188321. doi: 10.1128/spectrum.01883-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196784 Free PMC article.
-
Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production.J Virol. 2013 Dec;87(23):12814-27. doi: 10.1128/JVI.02355-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049179 Free PMC article.
-
Herpes Simplex Virus Type 1 Infection Disturbs the Mitochondrial Network, Leading to Type I Interferon Production through the RNA Polymerase III/RIG-I Pathway.mBio. 2021 Dec 21;12(6):e0255721. doi: 10.1128/mBio.02557-21. Epub 2021 Nov 23. mBio. 2021. PMID: 34809467 Free PMC article.
Cited by
-
The herpes simplex virus 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR.J Virol. 2013 Jan;87(2):859-71. doi: 10.1128/JVI.01158-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115300 Free PMC article.
-
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1.Microbiol Mol Biol Rev. 2020 Sep 30;84(4):e00099-20. doi: 10.1128/MMBR.00099-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 32998978 Free PMC article. Review.
-
The Triangle Relationship Between Long Noncoding RNA, RIG-I-like Receptor Signaling Pathway, and Glycolysis.Front Microbiol. 2021 Nov 30;12:807737. doi: 10.3389/fmicb.2021.807737. eCollection 2021. Front Microbiol. 2021. PMID: 34917069 Free PMC article. Review.
-
Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA.Mol Ther. 2021 Mar 3;29(3):1174-1185. doi: 10.1016/j.ymthe.2020.11.011. Epub 2020 Dec 21. Mol Ther. 2021. PMID: 33352107 Free PMC article.
-
Advanced progress in the genetic modification of the oncolytic HSV-1 virus.Front Oncol. 2025 Jan 21;14:1525940. doi: 10.3389/fonc.2024.1525940. eCollection 2024. Front Oncol. 2025. PMID: 39906660 Free PMC article. Review.
References
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

