HIV-1 virus-like particles bearing pure env trimers expose neutralizing epitopes but occlude nonneutralizing epitopes
- PMID: 22301141
- PMCID: PMC3302546
- DOI: 10.1128/JVI.06938-11
HIV-1 virus-like particles bearing pure env trimers expose neutralizing epitopes but occlude nonneutralizing epitopes
Abstract
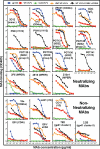
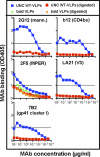
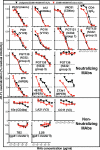
Hypothetically, since native HIV-1 Env trimers are exclusively recognized by neutralizing antibodies, they might induce the neutralizing antibodies in a vaccine setting. This idea has not been evaluated due to the difficulty of separating trimers from nonfunctional Env (uncleaved gp160 and gp41 stumps). The latter are immunodominant and induce nonneutralizing antibodies. We previously showed that nonfunctional Env can be selectively cleared from virus-like particle (VLP) surfaces by enzyme digests (E. T. Crooks, T. Tong(,) K. Osawa, and J. M. Binley, J.Virol. 85:5825, 2011). Here, we investigated the effects of these digests on the antigenicity of VLPs and their sensitivity to neutralization. Before digestion, WT VLPs (bearing wild-type Env) and UNC VLPs (bearing uncleaved gp160) were recognized by various Env-specific monoclonal antibodies (MAbs), irrespective of their neutralizing activity, a result which is consistent with the presence of nonfunctional Env. After digestion, only neutralizing MAbs recognized WT VLPs, consistent with selective removal of nonfunctional Env (i.e., "trimer VLPs"). Digests eliminated the binding of all MAbs to UNC VLPs, again consistent with removal of nonfunctional Env. An exception was MAb 2F5, which weakly bound to digested UNC VLPs and bald VLPs (bearing no Env), perhaps due to lipid cross-reactivity. Trimer VLPs were infectious, and their neutralization sensitivity was largely comparable to that of undigested WT VLPs. However, they were ∼100-fold more sensitive to the MAbs 4E10 and Z13e1, suggesting increased exposure of the gp41 base. Importantly, a scatterplot analysis revealed a strong correlation between MAb binding and neutralization of trimer VLPs. This suggests that trimer VLPs bear essentially pure native trimer that should allow its unfettered evaluation in a vaccine setting.
Figures
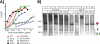





Similar articles
-
Inducible cell lines producing replication-defective human immunodeficiency virus particles containing envelope glycoproteins stabilized in a pretriggered conformation.J Virol. 2024 Dec 17;98(12):e0172024. doi: 10.1128/jvi.01720-24. Epub 2024 Nov 7. J Virol. 2024. PMID: 39508605 Free PMC article.
-
Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity.J Virol. 2015 Oct;89(20):10383-98. doi: 10.1128/JVI.01653-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246566 Free PMC article.
-
Identification of Novel Structural Determinants in MW965 Env That Regulate the Neutralization Phenotype and Conformational Masking Potential of Primary HIV-1 Isolates.J Virol. 2018 Feb 12;92(5):e01779-17. doi: 10.1128/JVI.01779-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237828 Free PMC article.
-
Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies.Retrovirology. 2018 Sep 12;15(1):63. doi: 10.1186/s12977-018-0445-y. Retrovirology. 2018. PMID: 30208933 Free PMC article. Review.
-
[Requirements for the Induction of Broadly Neutralizing Antibodies against HIV-1 by Vaccination].Mol Biol (Mosk). 2017 Nov-Dec;51(6):945-957. doi: 10.7868/S0026898417060076. Mol Biol (Mosk). 2017. PMID: 29271959 Review. Russian.
Cited by
-
Chemical Cross-Linking Stabilizes Native-Like HIV-1 Envelope Glycoprotein Trimer Antigens.J Virol. 2015 Oct 28;90(2):813-28. doi: 10.1128/JVI.01942-15. Print 2016 Jan 15. J Virol. 2015. PMID: 26512083 Free PMC article.
-
Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles.Nat Commun. 2016 Jun 28;7:12041. doi: 10.1038/ncomms12041. Nat Commun. 2016. PMID: 27349934 Free PMC article.
-
Structural Constraints at the Trimer Apex Stabilize the HIV-1 Envelope in a Closed, Antibody-Protected Conformation.mBio. 2018 Dec 11;9(6):e00955-18. doi: 10.1128/mBio.00955-18. mBio. 2018. PMID: 30538178 Free PMC article.
-
HIV-1 ENVELOPE. Effect of the cytoplasmic domain on antigenic characteristics of HIV-1 envelope glycoprotein.Science. 2015 Jul 10;349(6244):191-5. doi: 10.1126/science.aaa9804. Epub 2015 Jun 25. Science. 2015. PMID: 26113642 Free PMC article.
-
Probing the Structure of the HIV-1 Envelope Trimer Using Aspartate Scanning Mutagenesis.J Virol. 2020 Oct 14;94(21):e01426-20. doi: 10.1128/JVI.01426-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817217 Free PMC article.
References
-
- Binley J. June 2010. Enzymatic methods to produce pure native authentic HIV-1 Env trimer immunogens. US patent 61/360,067
-
- Binley JM. 2009. Specificities of broadly neutralizing anti-HIV-1 sera. Curr. Opin. HIV AIDS 4:364–372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

