A replication-incompetent PB2-knockout influenza A virus vaccine vector
- PMID: 22301144
- PMCID: PMC3318628
- DOI: 10.1128/JVI.06232-11
A replication-incompetent PB2-knockout influenza A virus vaccine vector
Abstract
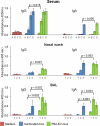
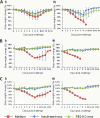
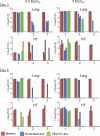
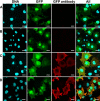
Vaccination is the primary form of protection from influenza virus infection. We recently developed a replication-incompetent PB2-knockout (PB2-KO) influenza virus that possesses a reporter gene (the green fluorescent protein gene) in the coding region of the PB2 segment. This virus replicated to high titers in PB2-expressing, but not unmodified, cells, suggesting its potential safety and feasibility as a vaccine. Here, we tested its efficacy in a murine model. The levels of IgG and IgA antibodies against influenza virus in sera, nasal washes, and bronchoalveolar lavage fluids of mice immunized with the PB2-KO virus were higher than those induced by a conventional inactivated vaccine. All PB2-KO virus-immunized mice survived challenges with lethal doses of influenza virus. Moreover, importantly, mice immunized with the PB2-KO virus produced antibodies against the reporter protein, suggesting that the PB2-KO virus has potential as a multivalent vaccine to combat infection with not only influenza virus but also other pathogens.
Figures
Comment in
-
Perspectives on replication-incompetent nasal influenza virus vaccines.Expert Rev Vaccines. 2012 Aug;11(8):907-9. doi: 10.1586/erv.12.64. Expert Rev Vaccines. 2012. PMID: 23002971
Similar articles
-
A replication-incompetent influenza virus bearing the HN glycoprotein of human parainfluenza virus as a bivalent vaccine.Vaccine. 2013 Dec 16;31(52):6239-46. doi: 10.1016/j.vaccine.2013.10.029. Epub 2013 Oct 19. Vaccine. 2013. PMID: 24144478 Free PMC article.
-
Evaluation of seasonal influenza vaccines for H1N1pdm09 and type B viruses based on a replication-incompetent PB2-KO virus.Vaccine. 2017 Apr 4;35(15):1892-1897. doi: 10.1016/j.vaccine.2017.02.041. Epub 2017 Mar 9. Vaccine. 2017. PMID: 28285982
-
A bivalent vaccine based on a replication-incompetent influenza virus protects against Streptococcus pneumoniae and influenza virus infection.J Virol. 2014 Nov;88(22):13410-7. doi: 10.1128/JVI.01205-14. Epub 2014 Sep 10. J Virol. 2014. PMID: 25210171 Free PMC article.
-
A novel bivalent vaccine based on a PB2-knockout influenza virus protects mice from pandemic H1N1 and highly pathogenic H5N1 virus challenges.J Virol. 2013 Jul;87(14):7874-81. doi: 10.1128/JVI.00076-13. Epub 2013 May 8. J Virol. 2013. PMID: 23658445 Free PMC article.
-
A Bivalent Vaccine Based on a PB2-Knockout Influenza Virus Protects Mice From Secondary Pneumococcal Pneumonia.J Infect Dis. 2015 Dec 15;212(12):1939-48. doi: 10.1093/infdis/jiv341. Epub 2015 Jun 29. J Infect Dis. 2015. PMID: 26123562 Free PMC article.
Cited by
-
Comparative Study of the Temperature Sensitive, Cold Adapted and Attenuated Mutations Present in the Master Donor Viruses of the Two Commercial Human Live Attenuated Influenza Vaccines.Viruses. 2019 Oct 10;11(10):928. doi: 10.3390/v11100928. Viruses. 2019. PMID: 31658679 Free PMC article.
-
A replication-incompetent influenza virus bearing the HN glycoprotein of human parainfluenza virus as a bivalent vaccine.Vaccine. 2013 Dec 16;31(52):6239-46. doi: 10.1016/j.vaccine.2013.10.029. Epub 2013 Oct 19. Vaccine. 2013. PMID: 24144478 Free PMC article.
-
H3N2 canine influenza virus-like particle vaccine with great protection in beagle dogs.Microbiol Spectr. 2024 Jun 14;12(8):e0044524. doi: 10.1128/spectrum.00445-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 38874403 Free PMC article.
-
Reverse Genetics Approaches for the Development of Influenza Vaccines.Int J Mol Sci. 2016 Dec 22;18(1):20. doi: 10.3390/ijms18010020. Int J Mol Sci. 2016. PMID: 28025504 Free PMC article. Review.
-
Influenza A Virus Agnostic Receptor Tropism Revealed Using a Novel Biological System with Terminal Sialic Acid Knockout Cells.J Virol. 2022 Aug 10;96(15):e0041622. doi: 10.1128/jvi.00416-22. Epub 2022 Jul 18. J Virol. 2022. PMID: 35862707 Free PMC article.
References
-
- Cox RJ, Brokstad KA, Ogra P. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 59:1–15 - PubMed
-
- Fiore AE, et al. 2010. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recommend. Rep. 59:1–62 - PubMed
-
- Halperin SA, et al. 2002. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 20:1240–1247 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

