Mouse norovirus 1 utilizes the cytoskeleton network to establish localization of the replication complex proximal to the microtubule organizing center
- PMID: 22301146
- PMCID: PMC3318650
- DOI: 10.1128/JVI.05784-11
Mouse norovirus 1 utilizes the cytoskeleton network to establish localization of the replication complex proximal to the microtubule organizing center
Abstract
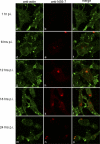
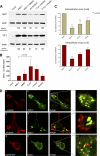
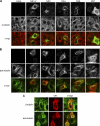
Human noroviruses (family Caliciviridae) are the leading cause of nonbacterial gastroenteritis worldwide. Although Human noroviruses are significant enteric pathogens, there exists no reliable vaccine or therapy to treat infected individuals. To date, attempts to cultivate Human noroviruses within the laboratory have met with little success; however, the related murine norovirus mouse norovirus 1 (MNV-1) has provided an ideal model system to study norovirus replication due to the ease with which the virus is cultivated and the ability to infect a small animal model with this virus. Previously we have identified the association between MNV-1 and components of the host secretory pathway and proposed a role for the viral open reading frame 1 proteins in the replication cycle. Here we describe for the first time a role for cytoskeletal components in early MNV-1 replication events. We show that the MNV-1 utilizes microtubules to position the replication complex adjacent to the microtubule organizing center. Chemical disruption of the microtubule network disperses the sites of MNV-1 replication throughout the cell and impairs production of viral protein and infectious virus. Furthermore, we demonstrate the ability of MNV-1 to redistribute acetylated tubulin to the replication complex and that this association is potentially mediated via the MNV-1 major structural protein, VP1. Transient expression of MNV-1 VP1 exhibited extensive colocalization with both α-tubulin and acetylated tubulin and was observed to alter the distribution of acetylated tubulin in transfected cells. This study highlights the role of the cytoskeleton in early virus replication events and demonstrates the importance of this interaction in establishing the intracellular location of MNV-1 replication complexes.
Figures





Similar articles
-
The Microtubule-Associated Innate Immune Sensor GEF-H1 Does Not Influence Mouse Norovirus Replication in Murine Macrophages.Viruses. 2019 Jan 10;11(1):47. doi: 10.3390/v11010047. Viruses. 2019. PMID: 30634661 Free PMC article.
-
The Norovirus NS3 Protein Is a Dynamic Lipid- and Microtubule-Associated Protein Involved in Viral RNA Replication.J Virol. 2017 Jan 18;91(3):e02138-16. doi: 10.1128/JVI.02138-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881660 Free PMC article.
-
Mouse norovirus replication is associated with virus-induced vesicle clusters originating from membranes derived from the secretory pathway.J Virol. 2009 Oct;83(19):9709-19. doi: 10.1128/JVI.00600-09. Epub 2009 Jul 8. J Virol. 2009. PMID: 19587041 Free PMC article.
-
Murine norovirus, a recently discovered and highly prevalent viral agent of mice.Lab Anim (NY). 2008 Jul;37(7):314-20. doi: 10.1038/laban0708-314. Lab Anim (NY). 2008. PMID: 18568010 Review.
-
Histologic Lesions Induced by Murine Norovirus Infection in Laboratory Mice.Vet Pathol. 2016 Jul;53(4):754-63. doi: 10.1177/0300985815618439. Epub 2016 Jan 20. Vet Pathol. 2016. PMID: 26792844 Free PMC article. Review.
Cited by
-
Bluetongue virus infection induces aberrant mitosis in mammalian cells.Virol J. 2013 Oct 28;10:319. doi: 10.1186/1743-422X-10-319. Virol J. 2013. PMID: 24165208 Free PMC article.
-
RNA Sequencing of Murine Norovirus-Infected Cells Reveals Transcriptional Alteration of Genes Important to Viral Recognition and Antigen Presentation.Front Immunol. 2017 Aug 11;8:959. doi: 10.3389/fimmu.2017.00959. eCollection 2017. Front Immunol. 2017. PMID: 28848558 Free PMC article.
-
Identification of RNA-protein interaction networks involved in the norovirus life cycle.J Virol. 2012 Nov;86(22):11977-90. doi: 10.1128/JVI.00432-12. Epub 2012 Aug 29. J Virol. 2012. PMID: 22933270 Free PMC article.
-
The Microtubule-Associated Innate Immune Sensor GEF-H1 Does Not Influence Mouse Norovirus Replication in Murine Macrophages.Viruses. 2019 Jan 10;11(1):47. doi: 10.3390/v11010047. Viruses. 2019. PMID: 30634661 Free PMC article.
-
Norovirus infection results in eIF2α independent host translation shut-off and remodels the G3BP1 interactome evading stress granule formation.PLoS Pathog. 2020 Jan 6;16(1):e1008250. doi: 10.1371/journal.ppat.1008250. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31905230 Free PMC article.
References
-
- Arce CA, Casale CH, Barra HS. 2008. Submembraneous microtubule cytoskeleton: regulation of ATPases by interaction with acetylated tubulin. FEBS J. 275:4664–4674 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

