The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR
- PMID: 22301152
- PMCID: PMC3318642
- DOI: 10.1128/JVI.05788-11
The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR
Abstract
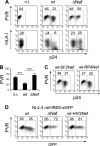
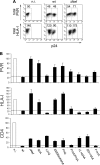
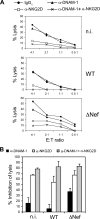
The human immunodeficiency virus type 1 (HIV-1) evades the immune responses of natural killer (NK) cells through mechanisms that have been partially deciphered. Here we show that in HIV-1-infected T lymphocytes, the early viral Nef protein downmodulates PVR (CD155, Necl-5), a ligand for the activating receptor DNAM-1 (CD226) expressed by all NK cells, CD8(+) T cells, and other cell types. This novel Nef activity is conserved by Nef proteins of laboratory HIV-1 strains (NL4-3, SF2) and of a patient-derived virus, but it is not maintained by HIV-2. Nef uses the same motifs to downregulate PVR and HLA-I molecules, likely by the same mechanisms. Indeed, as previously demonstrated for HLA-I, Nef reduces the total amounts of cell-associated PVR. Optimal downregulation of cell surface PVR by Nef also requires the presence of the late viral factor Vpu. In line with PVR reduction, the NK cell-mediated lysis of T cells infected by a wild-type but not Nef-deficient virus is virtually abrogated upon blocking of both DNAM-1 and another activating receptor, NKG2D, previously shown to mediate killing of HIV-infected cells. Together, these data demonstrate that the PVR downmodulation by Nef and Vpu is a strategy evolved by HIV-1 to prevent NK cell-mediated lysis of infected cells. The PVR downregulation reported here has the potential to affect the immune responses of other DNAM-1-positive cells besides NK cells and to alter multiple PVR-mediated cellular processes, such as adhesion and migration, and may thus greatly influence HIV-1 pathogenesis.
Figures


Similar articles
-
CD155 on HIV-Infected Cells Is Not Modulated by HIV-1 Vpu and Nef but Synergizes with NKG2D Ligands to Trigger NK Cell Lysis of Autologous Primary HIV-Infected Cells.AIDS Res Hum Retroviruses. 2017 Feb;33(2):93-100. doi: 10.1089/AID.2015.0375. Epub 2016 Jul 20. AIDS Res Hum Retroviruses. 2017. PMID: 27296670 Free PMC article.
-
Cell Surface Downregulation of NK Cell Ligands by Patient-Derived HIV-1 Vpu and Nef Alleles.J Acquir Immune Defic Syndr. 2016 May 1;72(1):1-10. doi: 10.1097/QAI.0000000000000917. J Acquir Immune Defic Syndr. 2016. PMID: 26656785
-
Upregulation of BST-2 by Type I Interferons Reduces the Capacity of Vpu To Protect HIV-1-Infected Cells from NK Cell Responses.mBio. 2019 Jun 18;10(3):e01113-19. doi: 10.1128/mBio.01113-19. mBio. 2019. PMID: 31213558 Free PMC article.
-
The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors.Immunol Rev. 1999 Apr;168:51-63. doi: 10.1111/j.1600-065x.1999.tb01282.x. Immunol Rev. 1999. PMID: 10399064 Review.
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
Cited by
-
Balancing natural killer cell activation through paired receptors.Nat Rev Immunol. 2015 Apr;15(4):243-54. doi: 10.1038/nri3799. Epub 2015 Mar 6. Nat Rev Immunol. 2015. PMID: 25743219 Review.
-
Efficient BST2 antagonism by Vpu is critical for early HIV-1 dissemination in humanized mice.Retrovirology. 2013 Nov 6;10:128. doi: 10.1186/1742-4690-10-128. Retrovirology. 2013. PMID: 24195843 Free PMC article.
-
Enhancing natural killer cell function with gp41-targeting bispecific antibodies to combat HIV infection.AIDS. 2020 Jul 15;34(9):1313-1323. doi: 10.1097/QAD.0000000000002543. AIDS. 2020. PMID: 32287071 Free PMC article.
-
Remodeling of the Host Cell Plasma Membrane by HIV-1 Nef and Vpu: A Strategy to Ensure Viral Fitness and Persistence.Viruses. 2016 Mar 3;8(3):67. doi: 10.3390/v8030067. Viruses. 2016. PMID: 26950141 Free PMC article. Review.
-
HIV-1 Vpu Downmodulates ICAM-1 Expression, Resulting in Decreased Killing of Infected CD4+ T Cells by NK Cells.J Virol. 2017 Mar 29;91(8):e02442-16. doi: 10.1128/JVI.02442-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148794 Free PMC article.
References
-
- Ardolino M, et al. 2011. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T-cell interaction. Blood 117:4778–4786 - PubMed
-
- Arien KK, Verhasselt B. 2008. HIV Nef: role in pathogenesis and viral fitness. Curr. HIV Res. 6:200–208 - PubMed
-
- Banning C, et al. 2010. A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells. PLoS One 5:e9344 doi:10.1371/journal.pone.0009344 - DOI - PMC - PubMed
-
- Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853–866 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

