The MC159 protein from the molluscum contagiosum poxvirus inhibits NF-κB activation by interacting with the IκB kinase complex
- PMID: 22301546
- PMCID: PMC3288875
- DOI: 10.4049/jimmunol.1100136
The MC159 protein from the molluscum contagiosum poxvirus inhibits NF-κB activation by interacting with the IκB kinase complex
Abstract
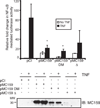
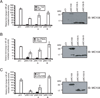
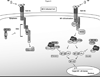
Molluscum contagiosum virus (MCV) causes persistent neoplasms in healthy and immunocompromised people. Its ability to persist likely is due to its arsenal of viral immunoevasion proteins. For example, the MCV MC159 protein inhibits TNF-R1-induced NF-κB activation and apoptosis. The MC159 protein is a viral FLIP and, as such, possesses two tandem death effector domains (DEDs). We show in this article that, in human embryonic kidney 293 T cells, the expression of wild-type MC159 or a mutant MC159 protein containing the first DED (MC159 A) inhibited TNF-induced NF-κB, or NF-κB activated by PMA or MyD88 overexpression, whereas a mutant protein lacking the first DED (MC159 B) did not. We hypothesized that the MC159 protein targeted the IκB kinase (IKK) complex to inhibit these diverse signaling events. Indeed, the MC159 protein, but not MC159 B, coimmunoprecipitated with IKKγ. MC159 coimmunoprecipitated with IKKγ when using mouse embryonic fibroblasts that lack either IKKα or IKKβ, suggesting that the MC159 protein interacted directly with IKKγ. MC159-IKKγ coimmunoprecipitations were detected during infection of cells with either MCV isolated from human lesions or with a recombinant MC159-expressing vaccinia virus. MC159 also interacts with TRAF2, a signaling molecule involved in NF-κB activation. However, mutational analysis of MC159 failed to reveal a correlation between MC159-TRAF2 interactions and MC159's inhibitory function. We propose that MC159-IKK interactions, but not MC159-TRAF2 interactions, are responsible for inhibiting NF-κB activation.
Figures






Similar articles
-
MC159 of Molluscum Contagiosum Virus Suppresses Autophagy by Recruiting Cellular SH3BP4 via an SH3 Domain-Mediated Interaction.J Virol. 2019 May 1;93(10):e01613-18. doi: 10.1128/JVI.01613-18. Print 2019 May 15. J Virol. 2019. PMID: 30842330 Free PMC article.
-
Molluscum Contagiosum Virus MC159 Abrogates cIAP1-NEMO Interactions and Inhibits NEMO Polyubiquitination.J Virol. 2017 Jul 12;91(15):e00276-17. doi: 10.1128/JVI.00276-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28515292 Free PMC article.
-
Functional Comparison of Molluscum Contagiosum Virus vFLIP MC159 with Murine Cytomegalovirus M36/vICA and M45/vIRA Proteins.J Virol. 2015 Dec 30;90(6):2895-905. doi: 10.1128/JVI.02729-15. J Virol. 2015. PMID: 26719271 Free PMC article.
-
Immune evasion strategies of molluscum contagiosum virus.Adv Virus Res. 2015;92:201-52. doi: 10.1016/bs.aivir.2014.11.004. Epub 2015 Jan 13. Adv Virus Res. 2015. PMID: 25701888 Review.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
MC159 of Molluscum Contagiosum Virus Suppresses Autophagy by Recruiting Cellular SH3BP4 via an SH3 Domain-Mediated Interaction.J Virol. 2019 May 1;93(10):e01613-18. doi: 10.1128/JVI.01613-18. Print 2019 May 15. J Virol. 2019. PMID: 30842330 Free PMC article.
-
Viral and cellular FLICE-inhibitory proteins: a comparison of their roles in regulating intrinsic immune responses.J Virol. 2014 Jun;88(12):6539-41. doi: 10.1128/JVI.00276-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719415 Free PMC article.
-
A parapoxviral virion protein inhibits NF-κB signaling early in infection.PLoS Pathog. 2017 Aug 7;13(8):e1006561. doi: 10.1371/journal.ppat.1006561. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28787456 Free PMC article.
-
Suppression of NF-κB Activity: A Viral Immune Evasion Mechanism.Viruses. 2018 Aug 4;10(8):409. doi: 10.3390/v10080409. Viruses. 2018. PMID: 30081579 Free PMC article. Review.
-
Molluscum Contagiosum Virus MC159 Abrogates cIAP1-NEMO Interactions and Inhibits NEMO Polyubiquitination.J Virol. 2017 Jul 12;91(15):e00276-17. doi: 10.1128/JVI.00276-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28515292 Free PMC article.
References
-
- Bugert JJ, Lohmuller C, Darai G. Characterization of early gene transcripts of molluscum contagiosum virus. Virology. 1999;257:119–129. - PubMed
-
- Senkevich TG, Bugert JJ, Sisler JR, Koonin EV, Darai G, Moss B. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science. 1996;273:813–816. - PubMed
-
- Senkevich TG, Koonin EV, Bugert JJ, Darai G, Moss B. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology. 1997;233:19–42. - PubMed
-
- Tyring SK. Molluscum contagiosum: the importance of early diagnosis and treatment. Am J Obstet Gynecol. 2003;189:S12–S16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

