The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis
- PMID: 22301556
- PMCID: PMC3457684
- DOI: 10.1126/scitranslmed.3003326
The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis
Abstract
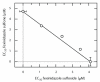
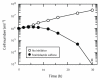
Safer and more effective oral drugs are required to treat visceral leishmaniasis, a parasitic disease that kills 50,000 to 60,000 people each year in parts of Asia, Africa, and Latin America. Here, we report that fexinidazole, a drug currently in phase 1 clinical trials for treating African trypanosomiasis, shows promise for treating visceral leishmaniasis. This 2-substituted 5-nitroimidazole drug is rapidly oxidized in vivo in mice, dogs, and humans to sulfoxide and sulfone metabolites. Both metabolites of fexinidazole were active against Leishmania donovani amastigotes grown in macrophages, whereas the parent compound was inactive. Pharmacokinetic studies with fexinidazole (200 mg/kg) showed that fexinidazole sulfone achieves blood concentrations in mice above the EC(99) (effective concentration inhibiting growth by 99%) value for at least 24 hours after a single oral dose. A once-daily regimen for 5 days at this dose resulted in a 98.4% suppression of infection in a mouse model of visceral leishmaniasis, equivalent to that seen with the drugs miltefosine and Pentostam, which are currently used clinically to treat this tropical disease. In African trypanosomes, the mode of action of nitro drugs involves reductive activation via a NADH (reduced form of nicotinamide adenine dinucleotide)-dependent bacterial-like nitroreductase. Overexpression of the leishmanial homolog of this nitroreductase in L. donovani increased sensitivity to fexinidazole by 19-fold, indicating that a similar mechanism is involved in both parasites. These findings illustrate the potential of fexinidazole as an oral drug therapy for treating visceral leishmaniasis.
Figures


References
-
- Raju TNK. The Nobel chronicles. Lancet. 2000;355:1022. - PubMed
-
- Matlashewski G, Arana B, Kroeger A, Battacharya S, Sundar S, Das P, Sinha PK, Rijal S, Mondal D, Zilberstein D, Alvar J. Visceral leishmaniasis: elimination with existing interventions. Lancet Infect. Dis. 2011;11:322–325. - PubMed
-
- World Health Organization . Control of the Leishmaniases 949. World Health Organization; Geneva: 2010. - PubMed
-
- den Boer ML, Alvar J, Davidson RN, Ritmeijer K, Balasegaram M. Developments in the treatment of visceral leishmaniasis. Expert Opinion on Emerging Drugs. 2009;14:395–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

