Pannexin protein expression in the rat middle cerebral artery
- PMID: 22301733
- PMCID: PMC3290039
- DOI: 10.1159/000332329
Pannexin protein expression in the rat middle cerebral artery
Abstract
Background: Connexin proteins are well known to participate in cell-to-cell communication within the cerebral vasculature. Pannexins are a recently discovered family of proteins that could potentially be involved in cell-to-cell communication. Herein, we sought to determine whether pannexins are expressed in rat middle cerebral artery (MCA).
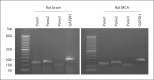
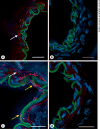
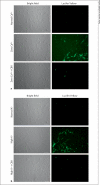
Methods: A combination of RT-PCR, immunoblotting and immunohistochemistry techniques was used to characterize the expression pattern of pannexins in rat MCA. A fluorescent dye uptake approach in cultured smooth muscle cells was used to determine whether these cells have functional hemichannels.
Results: We report for the first time that pannexins are expressed in the cerebral vasculature. We reveal that pannexin 1 is expressed in smooth muscle but not in endothelium and pannexin 2 is expressed in both endothelium and smooth muscle. Fluorescent dye entered cultured smooth muscle cells in the absence of extracellular calcium or when the cells were depolarized, which was prevented by the putative hemichannel blocker carbenoxolone.
Conclusions: The identification of pannexins in rat MCA indicates that pannexin expression is not restricted to neuronal cells. Dye uptake in cultured smooth muscle cells exhibited properties similar to those of connexin and pannexin hemichannels, which may represent another form of cell-to-cell communication within the vasculature.
Copyright © 2012 S. Karger AG, Basel.
Figures


References
-
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. - PubMed
-
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. - PubMed
-
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

