In vitro and in vivo investigation of dexibuprofen derivatives for CNS delivery
- PMID: 22301864
- PMCID: PMC4010340
- DOI: 10.1038/aps.2011.144
In vitro and in vivo investigation of dexibuprofen derivatives for CNS delivery
Abstract
Aim: Dexibuprofen, the S(+)-isomer of ibuprofen, is an effective therapeutic agent for the treatment of neurodegenerative disorders. However, its clinical use is hampered by a limited brain distribution. The aim of this study was to design and synthesize brain-targeting dexibuprofen prodrugs and to evaluate their brain-targeting efficiency using biodistribution and pharmacokinetic analysis.
Methods: In vitro stability, biodistribution and pharmacokinetic studies were performed on male Sprague-Dawley rats. The concentrations of dexibuprofen in biosamples, including the plasma, brain, heart, liver, spleen, lung, and kidney, were measured using high pressure lipid chromatography (HPLC). The pharmacokinetic parameters of the drug in the plasma and tissues were calculated using obtained data and statistics.
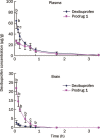
Results: Five dexibuprofen prodrugs that were modified to contain ethanolamine-related structures were designed and synthesized. Their chemical structures were confirmed using (1)H NMR, (13)C NMR, IR, and HRMS. In the biodistribution study, 10 min after intravenous administration of dexibuprofen (11.70 mg/kg) and its prodrugs (the dose of each compound was equivalent to 11.70 mg/kg of dexibuprofen) in male Sprague-Dawley rats, the dexibuprofen concentrations in the brain and plasma were measured. The C(brain)/C(plasma) ratios of prodrugs 1, 2, 3, 4, and 5 were 17.0-, 15.7-, 7.88-, 9.31-, and 3.42-fold higher than that of dexibuprofen, respectively (P<0.01). Thus, each of the prodrugs exhibited a significantly enhanced brain distribution when compared with dexibuprofen. In the pharmacokinetic study, prodrug 1 exhibited a brain-targeting index of 11.19 {DTI=(AUC(brain)/AUC(plasma))(1)/(AUC(brain)/AUC(plasma))(dexibuprofen)}.
Conclusion: The ethanolamine-related structures may play an important role in transport across the brain blood barrier.
Figures





Similar articles
-
Mechanism of brain targeting by dexibuprofen prodrugs modified with ethanolamine-related structures.J Cereb Blood Flow Metab. 2015 Dec;35(12):1985-94. doi: 10.1038/jcbfm.2015.160. Epub 2015 Jul 8. J Cereb Blood Flow Metab. 2015. PMID: 26154870 Free PMC article.
-
Synthesis, Bioevaluation and Molecular Dynamic Simulation Studies of Dexibuprofen-Antioxidant Mutual Prodrugs.Int J Mol Sci. 2016 Dec 21;17(12):2151. doi: 10.3390/ijms17122151. Int J Mol Sci. 2016. PMID: 28009827 Free PMC article.
-
Synthesis, in vitro and in vivo characterization of glycosyl derivatives of ibuprofen as novel prodrugs for brain drug delivery.J Drug Target. 2009 May;17(4):318-28. doi: 10.1080/10611860902795399. J Drug Target. 2009. PMID: 19558357
-
Prodrugs and endogenous transporters: are they suitable tools for drug targeting into the central nervous system?Curr Pharm Des. 2011;17(32):3560-76. doi: 10.2174/138161211798194486. Curr Pharm Des. 2011. PMID: 22074427 Review.
-
Ethanolamine: A Potential Promoiety with Additional Effects on the Brain.CNS Neurol Disord Drug Targets. 2022;21(2):108-117. doi: 10.2174/1871527319999201211204645. CNS Neurol Disord Drug Targets. 2022. PMID: 33319663 Review.
Cited by
-
Dexibuprofen ameliorates peripheral and central risk factors associated with Alzheimer's disease in metabolically stressed APPswe/PS1dE9 mice.Cell Biosci. 2021 Jul 22;11(1):141. doi: 10.1186/s13578-021-00646-w. Cell Biosci. 2021. PMID: 34294142 Free PMC article.
-
Mechanism of brain targeting by dexibuprofen prodrugs modified with ethanolamine-related structures.J Cereb Blood Flow Metab. 2015 Dec;35(12):1985-94. doi: 10.1038/jcbfm.2015.160. Epub 2015 Jul 8. J Cereb Blood Flow Metab. 2015. PMID: 26154870 Free PMC article.
-
Image-guided drug delivery to the brain using nanotechnology.Drug Discov Today. 2013 Nov;18(21-22):1074-80. doi: 10.1016/j.drudis.2013.06.010. Epub 2013 Jun 28. Drug Discov Today. 2013. PMID: 23817076 Free PMC article. Review.
-
Targeting brain cells with glutathione-modulated nanoliposomes: in vitro and in vivo study.Drug Des Devel Ther. 2015 Jul 20;9:3705-27. doi: 10.2147/DDDT.S85302. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26229435 Free PMC article.
-
Dexibuprofen prevents neurodegeneration and cognitive decline in APPswe/PS1dE9 through multiple signaling pathways.Redox Biol. 2017 Oct;13:345-352. doi: 10.1016/j.redox.2017.06.003. Epub 2017 Jun 15. Redox Biol. 2017. PMID: 28646794 Free PMC article.
References
-
- Patel PM, Drummond JC, Sano T, Cole DJ, Kalkman CJ, Yaksh TL. Effect of ibuprofen on regional eicosanoid production and neuronal injury after forebrain ischemia in rats. Brain Res. 1993;614:315–24. - PubMed
-
- Szekely CA, Thorne JE, Zandi PP, Messias E, Breitner JC, Goodman SN. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepidemiology. 2004;23:159–69. - PubMed
-
- Silakova JM, Hewett JA, Hewett SJ. Naproxen reduces excitotoxic neurodegeneration in vivo with an extended therapeutic window. J Pharmacol Exp Ther. 2004;309:1060–6. - PubMed
-
- Mannila A, Rautio J, Lehtonen M, Jarvinen T, Savolainen J. Inefficient central nervous system delivery limits the use of ibuprofen in neurodegenerative diseases. Eur J Pharm Sci. 2005;24:101–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

