Cbl exposes its RING finger
- PMID: 22301873
- PMCID: PMC6333313
- DOI: 10.1038/nsmb.2241
Cbl exposes its RING finger
Abstract
The Cbl family of RING finger ubiquitin ligases regulates signaling in many systems. Two new studies provide a structural basis for how phosphorylation of a specific tyrosine in the Cbl proteins enhances their ubiquitin ligase activity, giving insight into how ubiquitination by Cbl proteins is restricted to specific substrates.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures

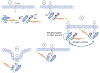
Comment on
-
Structural basis for autoinhibition and phosphorylation-dependent activation of c-Cbl.Nat Struct Mol Biol. 2012 Jan 22;19(2):184-92. doi: 10.1038/nsmb.2231. Nat Struct Mol Biol. 2012. PMID: 22266821 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

