Expression pattern and putative function of EXL1 and homologous genes in Arabidopsis
- PMID: 22301961
- PMCID: PMC3357360
- DOI: 10.4161/psb.7.1.18369
Expression pattern and putative function of EXL1 and homologous genes in Arabidopsis
Abstract
The Arabidopsis EXORDIUM-LIKE1 (EXL1) gene (At1g35140) is required for adaptation to carbon (C)- and energy-limiting growth conditions. An exl1 loss of function mutant showed diminished biomass production in a low total irradiance growth regime, impaired survival during extended night, and impaired survival of anoxia stress. We show here additional expression data and discuss the putative roles of EXL1. We hypothesize that EXL1 suppresses brassinosteroid-dependent growth and controls C allocation in the cell. In-depth expression analysis of homologous genes suggests that the EXL2 (At5g64260) and EXL4 (At5g09440) genes play similar roles.
Figures


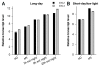

References
-
- Gout E, Bligny R, Douce R, Boisson A-M, Rivasseu C. Early response of plant cell to carbon deprivation: in vivo 31P-NMR spectroscopy shows a quasi-instantaneous disruption on cytosolic sugars, phosphorylated intermediates of energy metabolism, phosphate partitioning, and intracellular pHs. New Phytol. 2011;189:135–47. doi: 10.1111/j.1469-8137.2010.03449.x. - DOI - PubMed
-
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, et al. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004;39:847–62. doi: 10.1111/j.1365-313X.2004.02173.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
