Neural Regulation of the Stress Response: The Many Faces of Feedback
- PMID: 22302180
- PMCID: PMC3956711
- DOI: 10.1007/s10571-012-9801-y
Neural Regulation of the Stress Response: The Many Faces of Feedback
Abstract
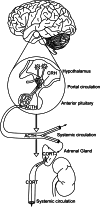
The mammalian stress response is an integrated physiological and psychological reaction to real or perceived adversity. Glucocorticoids (GCs) are an important component of this response, acting to redistribute energy resources to both optimize survival in the face of challenge and restore homeostasis after the immediate threat has subsided. Release of GCs is mediated by the hypothalamo-pituitary-adrenocortical (HPA) axis, driven by a neural signal originating in the paraventricular nucleus (PVN). Stress levels of GCs bind to glucocorticoid receptors (GRs) in multiple body compartments, including brain, and consequently have wide-reaching actions. For this reason, GCs serve a vital function in feedback inhibition of their own secretion. Fast, non-genomic feedback inhibition of the HPA axis is mediated at least in part by GC signaling in the PVN, acting by a cannabinoid-dependent mechanism to rapidly reduce both neural activity and GC release. Delayed feedback termination of the HPA axis response is mediated by forebrain GRs, presumably by genomic mechanisms. GCs also act in the brainstem to attenuate neuropeptidergic excitatory input to the PVN via acceleration of mRNA degradation, providing a mechanism to attenuate future responses to stressors. Thus, rather than having a single defined feedback switch, GCs work through multiple neurocircuits and signaling mechanisms to coordinate HPA axis activity to suit the overall needs of multiple body systems.
Figures



Similar articles
-
Neural regulation of the stress response: glucocorticoid feedback mechanisms.Braz J Med Biol Res. 2012 Apr;45(4):292-8. doi: 10.1590/s0100-879x2012007500041. Epub 2012 Mar 29. Braz J Med Biol Res. 2012. PMID: 22450375 Free PMC article. Review.
-
Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture.Stress. 2020 Nov;23(6):617-632. doi: 10.1080/10253890.2020.1859475. Epub 2020 Dec 21. Stress. 2020. PMID: 33345670 Free PMC article. Review.
-
Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic-pituitary-adrenal axis is dependent upon stressor specific neurocircuitry.Brain Res. 2000 Jul 7;870(1-2):87-101. doi: 10.1016/s0006-8993(00)02405-7. Brain Res. 2000. PMID: 10869505
-
Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response.Compr Physiol. 2016 Mar 15;6(2):603-21. doi: 10.1002/cphy.c150015. Compr Physiol. 2016. PMID: 27065163 Free PMC article. Review.
-
Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling.Endocrinology. 2010 Oct;151(10):4811-9. doi: 10.1210/en.2010-0285. Epub 2010 Aug 11. Endocrinology. 2010. PMID: 20702575 Free PMC article.
Cited by
-
Stress, a Brief Update.Int J Psychol Res (Medellin). 2023 Oct 10;16(2):105-121. doi: 10.21500/20112084.5815. eCollection 2023 Jul-Dec. Int J Psychol Res (Medellin). 2023. PMID: 38106958 Free PMC article.
-
Adipocyte glucocorticoid receptors mediate fat-to-brain signaling.Psychoneuroendocrinology. 2015 Jun;56:110-9. doi: 10.1016/j.psyneuen.2015.03.008. Epub 2015 Mar 12. Psychoneuroendocrinology. 2015. PMID: 25808702 Free PMC article.
-
Autonomic Neural Circuit and Intervention for Comorbidity Anxiety and Cardiovascular Disease.Front Physiol. 2022 Apr 27;13:852891. doi: 10.3389/fphys.2022.852891. eCollection 2022. Front Physiol. 2022. PMID: 35574459 Free PMC article. Review.
-
Minimum carbon dioxide is a key predictor of the respiratory health of pigs in climate-controlled housing systems.Porcine Health Manag. 2024 Dec 20;10(1):59. doi: 10.1186/s40813-024-00408-3. Porcine Health Manag. 2024. PMID: 39707558 Free PMC article.
-
Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys.Horm Behav. 2013 Apr;63(4):646-58. doi: 10.1016/j.yhbeh.2013.01.010. Epub 2013 Feb 1. Horm Behav. 2013. PMID: 23380162 Free PMC article.
References
-
- Adam TC, Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91:449–458 - PubMed
-
- Bartanusz V, Muller D, Gaillard RC, Streit P, Vutskits L, Kiss JZ (2004) Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. Eur J Neurosci 19:777–782 - PubMed
-
- Beaulieu S, Di Paolo T, Barden N (1986) Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology 44:247–254 - PubMed
-
- Beaulieu S, Di Paolo T, Cote J, Barden N (1987) Participation of the central amygdaloid nucleus in the response of adrenocorticotropin secretion to immobilization stress: opposing roles of the noradrenergic and dopaminergic systems. Neuroendocrinology 45:37–46 - PubMed
-
- Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF (2001) Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol 21:516–521 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

