Long-term blood pressure control: is there a set-point in the brain?
- PMID: 22302247
- PMCID: PMC10717488
- DOI: 10.1007/s12576-012-0192-0
Long-term blood pressure control: is there a set-point in the brain?
Abstract
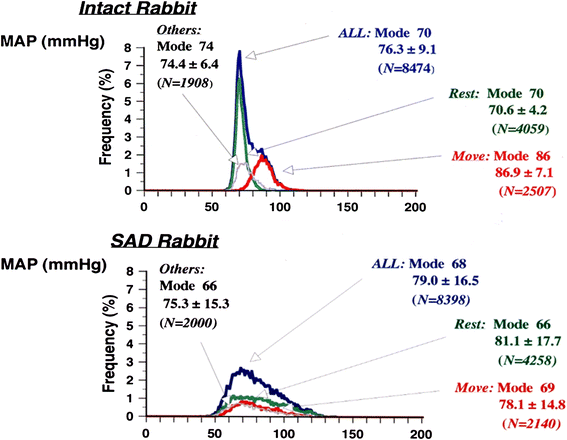
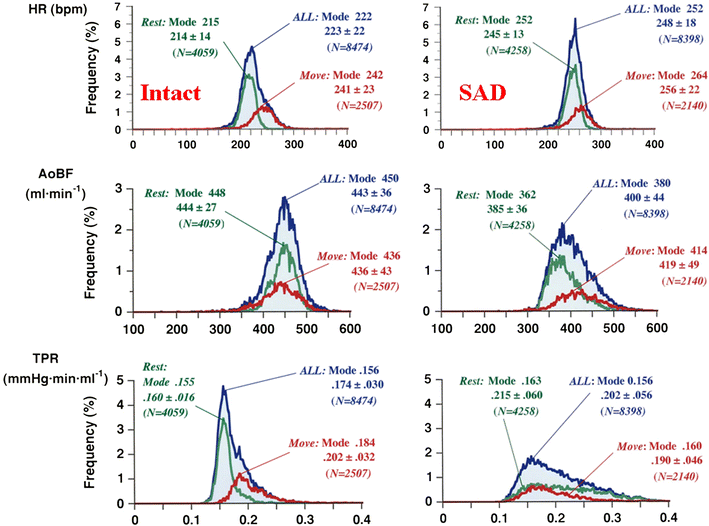
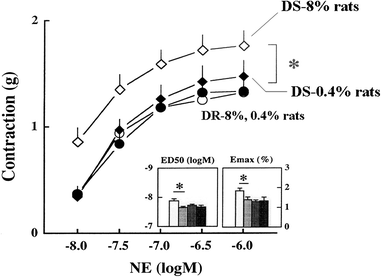
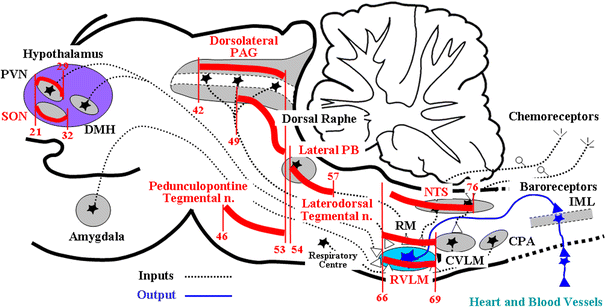
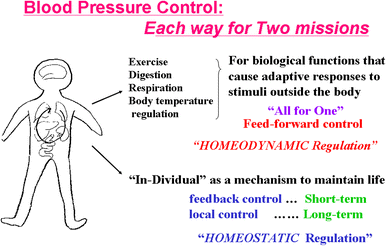
Mean arterial pressure fluctuates depending on physical or psychological activity, but should be stable at rest at around 100 mmHg throughout an entire life in human. The causes of hypertension and the blood pressure regulation mechanisms have been discussed for a long time, and many aspects have recently become more clear. Circulatory shock or short-term hypotension can be treated based on what is now known, but chronic hypertension is still difficult to treat thoroughly. The exact mechanisms for long-term blood pressure regulation have yet not been elucidated. Neuro–humoral interaction has been suggested as one of the mechanisms. Then, from the 1990s, paracrine hormones like nitric oxide or endothelins have been extensively researched in order to develop endothelial local control mechanisms for blood pressure, which have some relationships to long-term control. Although these new ideas and mechanisms are newly developed, no clear explanation for long-term control has yet been discussed, except for renal abnormality. Recently, a central set-point theory has begun to be discussed. This review will discuss the mechanisms for long-term blood pressure control, based on putative biological missions of circulatory function for life support.
Figures












Similar articles
-
Chronic salt loading and cardiovascular-associated changes in experimental diabetes in rats.Clin Exp Pharmacol Physiol. 2007 Jul;34(7):574-80. doi: 10.1111/j.1440-1681.2007.04625.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17581211
-
Central and peripheral vasopressin interact differently with sympathetic nervous system and renin-angiotensin system in renal hypertensive rabbits.Circ Res. 1993 Jun;72(6):1255-65. doi: 10.1161/01.res.72.6.1255. Circ Res. 1993. PMID: 8495554
-
Post-exercise elevations in sympathetic nerve activity and baroreflex function in normotensive rabbits.Clin Exp Hypertens. 2000 May;22(4):431-44. doi: 10.1081/ceh-100100082. Clin Exp Hypertens. 2000. PMID: 10830754
-
A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex.Am J Physiol Regul Integr Comp Physiol. 2005 Apr;288(4):R846-55. doi: 10.1152/ajpregu.00474.2004. Am J Physiol Regul Integr Comp Physiol. 2005. PMID: 15793038 Review.
-
Baroreflexes and cardiovascular regulation in hypertension.J Cardiovasc Pharmacol. 1995;26 Suppl 2:S7-16. J Cardiovasc Pharmacol. 1995. PMID: 8642810 Review.
Cited by
-
Chemoreflexes, sleep apnea, and sympathetic dysregulation.Curr Hypertens Rep. 2014 Sep;16(9):476. doi: 10.1007/s11906-014-0476-2. Curr Hypertens Rep. 2014. PMID: 25097113 Free PMC article. Review.
-
Apneic Sleep, Insufficient Sleep, and Hypertension.Hypertension. 2019 Apr;73(4):744-756. doi: 10.1161/HYPERTENSIONAHA.118.11780. Hypertension. 2019. PMID: 30776972 Free PMC article. Review. No abstract available.
-
The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation.Life (Basel). 2021 Sep 20;11(9):986. doi: 10.3390/life11090986. Life (Basel). 2021. PMID: 34575135 Free PMC article. Review.
-
Heart rate variability in people with multiple sclerosis: A systematic review and meta-analysis.Acta Neurol Belg. 2025 Jun 30. doi: 10.1007/s13760-025-02829-5. Online ahead of print. Acta Neurol Belg. 2025. PMID: 40587005 Review.
-
Time Restricted Feeding to the Light Cycle Dissociates Canonical Circadian Clocks and Physiological Rhythms in Heart Rate.Front Pharmacol. 2022 May 12;13:910195. doi: 10.3389/fphar.2022.910195. eCollection 2022. Front Pharmacol. 2022. PMID: 35645828 Free PMC article. Review.
References
-
- Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

