A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes
- PMID: 22302686
- PMCID: PMC3338659
- DOI: 10.1095/biolreprod.111.097253
A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes
Abstract
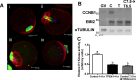
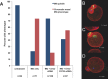
Precise coordination of meiotic progression is a critical determinant of an egg's capacity to be fertilized successfully, and zinc has emerged as a key regulatory element in this process. An early manifestation of a regulatory role for this transition metal is the significant increase in total intracellular zinc. This accumulation is essential for meiotic progression beyond telophase I and the establishment of meiotic arrest at metaphase II. The subsequent developmental event, fertilization, induces a rapid expulsion of labile zinc that is a hallmark event in meiotic resumption. In the present study, we show that the zinc fluxes work, in part, by altering the activity of the cytostatic factor (CSF), the cellular activity required for the establishment and maintenance of metaphase II arrest in the mature, unfertilized egg. We propose a model in which zinc exerts concentration-dependent regulation of meiosis through the CSF component EMI2, a zinc-binding protein. Together, the data support the conclusion that zinc itself, through its interaction with EMI2, is a central component of the CSF.
Figures





References
-
- Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001; 292: 2488 2492 - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003; 4: 517 529 - PubMed
-
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260: 3440 3450 - PubMed
-
- Poenie M, Alderton J, Tsien RY, Steinhardt RA. Changes of free calcium levels with stages of the cell division cycle. Nature 1985; 315: 147 149 - PubMed
-
- Krezel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem 2006; 11: 1049 1062 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

