Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice
- PMID: 22302798
- PMCID: PMC3307392
- DOI: 10.1523/JNEUROSCI.5123-11.2012
Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice
Abstract
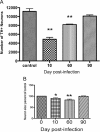
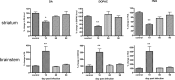
The A/VN/1203/04 strain of the H5N1 influenza virus is capable of infecting the CNS of mice and inducing a number of neurodegenerative pathologies. Here, we examined the effects of H5N1 on several pathological aspects affected in parkinsonism, including loss of the phenotype of dopaminergic neurons located in the substantia nigra pars compacta (SNpc), expression of monoamines and indolamines in brain, alterations in SNpc microglia number and morphology, and expression of cytokines, chemokines, and growth factors. We find that H5N1 induces a transient loss of the dopaminergic phenotype in SNpc and now report that this loss recovers by 90 d after infection. A similar pattern of loss and recovery was seen in monoamine levels of the basal ganglia. The inflammatory response in lung and different regions of the brain known to be targets of the H5N1 virus (brainstem, substantia nigra, striatum, and cortex) were examined at 3, 10, 21, 60, and 90 d after infection. In each of these brain regions, we found a significant increase in the number of activated microglia that lasted at least 90 d. We also quantified expression of IL-1α, IL-1β, IL-2, IL-6, IL-9, IL-10, IL-12(p70), IL-13, TNF-α, IFN-γ, granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, macrophage colony-stimulating factor, eotaxin, interferon-inducible protein 10, cytokine-induced neutrophil chemoattractant, monocyte chemotactic protein-1, macrophage inflammatory protein (MIP) 1α, MIP-1β, and VEGF, and found that the pattern and levels of expression are dependent on both brain region and time after infection. We conclude that H5N1 infection in mice induces a long-lasting inflammatory response in brain and may play a contributing factor in the development of pathologies in neurodegenerative disorders.
Figures




References
-
- Bazzu G, Calia G, Puggioni G, Spissu Y, Rocchitta G, Debetto P, Grigoletto J, Zusso M, Migheli R, Serra PA, Desole MS, Miele E. alpha-Synuclein- and MPTP-generated rodent models of Parkinson's disease and the study of extracellular striatal dopamine dynamics: a microdialysis approach. CNS Neurol Disord Drug Targets. 2010;9:482–490. - PubMed
-
- Boyd JD, Jang H, Shepherd KR, Faherty C, Slack S, Jiao Y, Smeyne RJ. Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction before loss of neurons in the substantia nigra pars compacta. Brain Res. 2007;1175:107–116. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
