Changing microcircuits in the subplate of the developing cortex
- PMID: 22302801
- PMCID: PMC3517995
- DOI: 10.1523/JNEUROSCI.4748-11.2012
Changing microcircuits in the subplate of the developing cortex
Abstract
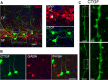
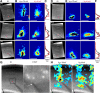
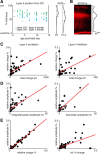
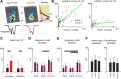
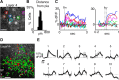
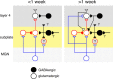
Subplate neurons (SPNs) are a population of neurons in the mammalian cerebral cortex that exist predominantly in the prenatal and early postnatal period. Loss of SPNs prevents the functional maturation of the cerebral cortex. SPNs receive subcortical input from the thalamus and relay this information to the developing cortical plate and thereby can influence cortical activity in a feedforward manner. Little is known about potential feedback projections from the cortical plate to SPNs. Thus, we investigated the spatial distribution of intracortical synaptic inputs to SPNs in vitro in mouse auditory cortex by photostimulation. We find that SPNs fell into two broad classes based on their distinct spatial patterns of synaptic inputs. The first class of SPNs receives inputs from only deep cortical layers, while the second class of SPNs receives inputs from deep as well as superficial layers including layer 4. We find that superficial cortical inputs to SPNs emerge in the second postnatal week and that SPNs that receive superficial cortical input are located more superficially than those that do not. Our data thus suggest that distinct circuits are present in the subplate and that, while SPNs participate in an early feedforward circuit, they are also involved in a feedback circuit at older ages. Together, our results show that SPNs are tightly integrated into the developing thalamocortical and intracortical circuit. The feedback projections from the cortical plate might enable SPNs to amplify thalamic inputs to SPNs.
Figures



References
-
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
