Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling
- PMID: 22302810
- PMCID: PMC6703370
- DOI: 10.1523/JNEUROSCI.5531-11.2012
Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling
Abstract
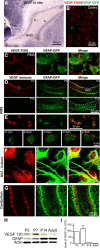
New neurons are constantly being generated in the postnatal subventricular zone. They have to migrate long distances via the rostral migratory stream (RMS) to reach their final destination in the olfactory bulb (OB). In adults, these neuronal precursors migrate in chains, ensheathed by astrocytic processes, and travel toward the OB along blood vessels (BVs) that topographically outline the RMS. The molecular and cellular mechanisms leading to the development of the RMS and the formation of the migration-promoting vasculature scaffold in the adult mice remain unclear. We now reveal that astrocytes orchestrate the formation and structural reorganization of the vasculature scaffold in the RMS and, during early developmental stages, the RMS contains only a few BVs oriented randomly with respect to the migrating neuroblasts. The first parallel BVs appeared at the outer border of the RMS, where vascular endothelial growth factor (VEGF)-expressing astrocytes are located. Gain-of-function and loss-of-function experiments revealed that astrocyte-derived VEGF plays a crucial role in the formation and growth of new BVs. Real-time videoimaging also showed that the migration of neuronal precursors in the developing RMS differs substantially from neuronal displacement in the adult migratory stream partially because of not yet fully developed vasculature scaffold. The downregulation of VEGF in vivo, specifically in the astrocytes of the developing RMS, affected the development of the vasculature scaffold and led to alterations in neuroblast migration. Altogether, our results demonstrate that astrocytes orchestrate the formation and growth of parallel BVs, crucial migration-promoting scaffolds in the adult migratory stream, via VEGF signaling.
Figures







References
-
- Alves JA, Barone P, Engelender S, Fróes MM, Menezes JR. Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. J Neurobiol. 2002;52:251–265. - PubMed
-
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. - PubMed
-
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/ TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26:1780–1790. - PubMed
-
- Colín-Castelán D, Phillips-Farfán BV, Gutiérrez-Ospina G, Fuentes-Farias AL, Báez-Saldaña A, Padilla-Cortés P, Meléndez-Herrera E. EphB4 is developmentally and differentially regulated in blood vessels throughout the forebrain neurogenic niche in the mouse brain: implications for vascular remodeling. Brain Res. 2011;1383:90–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
