NF-κB, the first quarter-century: remarkable progress and outstanding questions
- PMID: 22302935
- PMCID: PMC3278889
- DOI: 10.1101/gad.183434.111
NF-κB, the first quarter-century: remarkable progress and outstanding questions
Abstract
The ability to sense and adjust to the environment is crucial to life. For multicellular organisms, the ability to respond to external changes is essential not only for survival but also for normal development and physiology. Although signaling events can directly modify cellular function, typically signaling acts to alter transcriptional responses to generate both transient and sustained changes. Rapid, but transient, changes in gene expression are mediated by inducible transcription factors such as NF-κB. For the past 25 years, NF-κB has served as a paradigm for inducible transcription factors and has provided numerous insights into how signaling events influence gene expression and physiology. Since its discovery as a regulator of expression of the κ light chain gene in B cells, research on NF-κB continues to yield new insights into fundamental cellular processes. Advances in understanding the mechanisms that regulate NF-κB have been accompanied by progress in elucidating the biological significance of this transcription factor in various physiological processes. NF-κB likely plays the most prominent role in the development and function of the immune system and, not surprisingly, when dysregulated, contributes to the pathophysiology of inflammatory disease. As our appreciation of the fundamental role of inflammation in disease pathogenesis has increased, so too has the importance of NF-κB as a key regulatory molecule gained progressively greater significance. However, despite the tremendous progress that has been made in understanding the regulation of NF-κB, there is much that remains to be understood. In this review, we highlight both the progress that has been made and the fundamental questions that remain unanswered after 25 years of study.
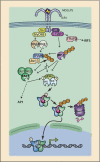
Figures






References
-
- Agou F, Ye F, Goffinont S, Courtois G, Yamaoka S, Israel A, Veron M 2002. NEMO trimerizes through its coiled-coil C-terminal domain. J Biol Chem 277: 17464–17475 - PubMed
-
- Agou F, Traincard F, Vinolo E, Courtois G, Yamaoka S, Israel A, Veron M 2004. The trimerization domain of NEMO is comprised of the interacting C-terminal CC2 and LZ coiled-coil subdomains. J Biol Chem. 279: 27861–27869 - PubMed
-
- Albertella MR, Campbell RD 1994. Characterization of a novel gene in the human major histocompatibility complex that encodes a potential new member of the IκB family of proteins. Hum Mol Genet 3: 793–799 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
