Structural basis of agrin-LRP4-MuSK signaling
- PMID: 22302937
- PMCID: PMC3278892
- DOI: 10.1101/gad.180885.111
Structural basis of agrin-LRP4-MuSK signaling
Abstract
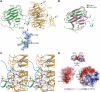
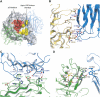
Synapses are the fundamental units of neural circuits that enable complex behaviors. The neuromuscular junction (NMJ), a synapse formed between a motoneuron and a muscle fiber, has contributed greatly to understanding of the general principles of synaptogenesis as well as of neuromuscular disorders. NMJ formation requires neural agrin, a motoneuron-derived protein, which interacts with LRP4 (low-density lipoprotein receptor-related protein 4) to activate the receptor tyrosine kinase MuSK (muscle-specific kinase). However, little is known of how signals are transduced from agrin to MuSK. Here, we present the first crystal structure of an agrin-LRP4 complex, consisting of two agrin-LRP4 heterodimers. Formation of the initial binary complex requires the z8 loop that is specifically present in neuronal, but not muscle, agrin and that promotes the synergistic formation of the tetramer through two additional interfaces. We show that the tetrameric complex is essential for neuronal agrin-induced acetylcholine receptor (AChR) clustering. Collectively, these results provide new insight into the agrin-LRP4-MuSK signaling cascade and NMJ formation and represent a novel mechanism for activation of receptor tyrosine kinases.
Figures




References
-
- Bourhis E, Wang W, Tam C, Hwang J, Zhang Y, Spittler D, Huang OW, Gong Y, Estevez A, Zilberleyb I, et al. 2011. Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure 19: 1433–1442 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
