Bacterial degradation of benzoate: cross-regulation between aerobic and anaerobic pathways
- PMID: 22303008
- PMCID: PMC3322966
- DOI: 10.1074/jbc.M111.309005
Bacterial degradation of benzoate: cross-regulation between aerobic and anaerobic pathways
Abstract
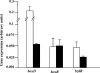
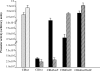
We have studied for the first time the transcriptional regulatory circuit that controls the expression of the box genes encoding the aerobic hybrid pathway used to assimilate benzoate via coenzyme A (CoA) derivatives in bacteria. The promoters responsible for the expression of the box cluster in the β-proteobacterium Azoarcus sp., their cognate transcriptional repressor, the BoxR protein, and the inducer molecule (benzoyl-CoA) have been characterized. The BoxR protein shows a significant sequence identity to the BzdR transcriptional repressor that controls the bzd genes involved in the anaerobic degradation of benzoate. Because the boxR gene is present in all box clusters so far identified in bacteria, the BoxR/benzoyl-CoA regulatory system appears to be a widespread strategy to control this aerobic hybrid pathway. Interestingly, the paralogous BoxR and BzdR regulators act synergistically to control the expression of the box and bzd genes. This cross-regulation between anaerobic and aerobic pathways for the catabolism of aromatic compounds has never been shown before, and it may reflect a biological strategy to increase the cell fitness in organisms that survive in environments subject to changing oxygen concentrations.
Figures




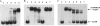


Similar articles
-
New insights into the BzdR-mediated transcriptional regulation of the anaerobic catabolism of benzoate in Azoarcus sp. CIB.Microbiology (Reading). 2008 Jan;154(Pt 1):306-316. doi: 10.1099/mic.0.2007/011361-0. Microbiology (Reading). 2008. PMID: 18174149
-
The bzd gene cluster, coding for anaerobic benzoate catabolism, in Azoarcus sp. strain CIB.J Bacteriol. 2004 Sep;186(17):5762-74. doi: 10.1128/JB.186.17.5762-5774.2004. J Bacteriol. 2004. PMID: 15317781 Free PMC article.
-
Further Insights into the Architecture of the PN Promoter That Controls the Expression of the bzd Genes in Azoarcus.Genes (Basel). 2019 Jun 27;10(7):489. doi: 10.3390/genes10070489. Genes (Basel). 2019. PMID: 31252700 Free PMC article.
-
BzdR, a repressor that controls the anaerobic catabolism of benzoate in Azoarcus sp. CIB, is the first member of a new subfamily of transcriptional regulators.J Biol Chem. 2005 Mar 18;280(11):10683-94. doi: 10.1074/jbc.M412259200. Epub 2005 Jan 5. J Biol Chem. 2005. PMID: 15634675
-
The bacterial degradation of benzoic acid and benzenoid compounds under anaerobic conditions: unifying trends and new perspectives.FEMS Microbiol Rev. 1994 Apr;13(4):441-68. doi: 10.1111/j.1574-6976.1994.tb00061.x. FEMS Microbiol Rev. 1994. PMID: 8011356 Review.
Cited by
-
Comparing Sediment Microbiomes in Contaminated and Pristine Wetlands along the Coast of Yucatan.Microorganisms. 2021 Apr 20;9(4):877. doi: 10.3390/microorganisms9040877. Microorganisms. 2021. PMID: 33923859 Free PMC article.
-
Metagenomic Profiling of Ocular Surface Microbiome Changes in Meibomian Gland Dysfunction.Invest Ophthalmol Vis Sci. 2020 Jul 1;61(8):22. doi: 10.1167/iovs.61.8.22. Invest Ophthalmol Vis Sci. 2020. PMID: 32673387 Free PMC article.
-
Metatranscriptome Analysis Deciphers Multifunctional Genes and Enzymes Linked With the Degradation of Aromatic Compounds and Pesticides in the Wheat Rhizosphere.Front Microbiol. 2018 Jul 3;9:1331. doi: 10.3389/fmicb.2018.01331. eCollection 2018. Front Microbiol. 2018. PMID: 30034370 Free PMC article.
-
A benzene-degrading nitrate-reducing microbial consortium displays aerobic and anaerobic benzene degradation pathways.Sci Rep. 2018 Mar 14;8(1):4490. doi: 10.1038/s41598-018-22617-x. Sci Rep. 2018. PMID: 29540736 Free PMC article.
-
Motility, Adhesion and c-di-GMP Influence the Endophytic Colonization of Rice by Azoarcus sp. CIB.Microorganisms. 2021 Mar 8;9(3):554. doi: 10.3390/microorganisms9030554. Microorganisms. 2021. PMID: 33800326 Free PMC article.
References
-
- Lovley D. R. (2003) Cleaning up with genomics. Applying molecular biology to bioremediation. Nat. Rev. Microbiol. 1, 35–44 - PubMed
-
- Díaz E. (2004) Bacterial degradation of aromatic pollutants. A paradigm of metabolic versatility. Int. Microbiol. 7, 173–180 - PubMed
-
- Fuchs G. (2008) Anaerobic metabolism of aromatic compounds. Ann. N.Y. Acad. Sci. 1125, 82–99 - PubMed
-
- Parales R. E., Resnick S. M. (2006) in Pseudomonas Molecular Biology of Emerging Issues (Ramos J. L., Levesque R. C., eds) Vol. 4, pp. 287–340, Springer, The Netherlands
Publication types
MeSH terms
Substances
Associated data
- Actions

