Alternating access to the transmembrane domain of the ATP-binding cassette protein cystic fibrosis transmembrane conductance regulator (ABCC7)
- PMID: 22303012
- PMCID: PMC3323033
- DOI: 10.1074/jbc.M112.342972
Alternating access to the transmembrane domain of the ATP-binding cassette protein cystic fibrosis transmembrane conductance regulator (ABCC7)
Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is a member of the ATP-binding cassette (ABC) protein family, most members of which act as active transporters. Actively transporting ABC proteins are thought to alternate between "outwardly facing" and "inwardly facing" conformations of the transmembrane substrate pathway. In CFTR, it is assumed that the outwardly facing conformation corresponds to the channel open state, based on homology with other ABC proteins. We have used patch clamp recording to quantify the rate of access of cysteine-reactive probes to cysteines introduced into two different transmembrane regions of CFTR from both the intracellular and extracellular solutions. Two probes, the large [2-sulfonatoethyl]methanethiosulfonate (MTSES) molecule and permeant Au(CN)(2)(-) ions, were applied to either side of the membrane to modify cysteines substituted for Leu-102 (first transmembrane region) and Thr-338 (sixth transmembrane region). Channel opening and closing were altered by mutations in the nucleotide binding domains of the channel. We find that, for both MTSES and Au(CN)(2)(-), access to these two cysteines from the cytoplasmic side is faster in open channels, whereas access to these same sites from the extracellular side is faster in closed channels. These results are consistent with alternating access to the transmembrane regions, however with the open state facing inwardly and the closed state facing outwardly. Our findings therefore prompt revision of current CFTR structural and mechanistic models, as well as having broader implications for transport mechanisms in all ABC proteins. Our results also suggest possible locations of both functional and dysfunctional ("vestigial") gates within the CFTR permeation pathway.
Figures
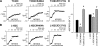
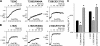





References
-
- DeFelice L. J., Goswami T. (2007) Transporters as channels. Annu. Rev. Physiol. 69, 87–112 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

