Interconnection of salt-induced hydrophobic compaction and secondary structure formation depends on solution conditions: revisiting early events of protein folding at single molecule resolution
- PMID: 22303014
- PMCID: PMC3322834
- DOI: 10.1074/jbc.M111.315648
Interconnection of salt-induced hydrophobic compaction and secondary structure formation depends on solution conditions: revisiting early events of protein folding at single molecule resolution
Abstract
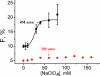
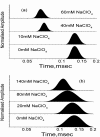
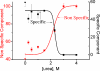
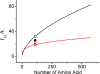
What happens in the early stage of protein folding remains an interesting unsolved problem. Rapid kinetics measurements with cytochrome c using submillisecond continuous flow mixing devices suggest simultaneous formation of a compact collapsed state and secondary structure. These data seem to indicate that collapse formation is guided by specific short and long range interactions (heteropolymer collapse). A contrasting interpretation also has been proposed, which suggests that the collapse formation is rapid, nonspecific, and a trivial solvent related compaction, which could as well be observed by a homopolymer (homopolymer collapse). We address this controversy using fluorescence correlation spectroscopy (FCS), which enables us to monitor the salt-induced compaction accompanying collapse formation and the associated time constant directly at single molecule resolution. In addition, we follow the formation of secondary structure using far UV CD. The data presented here suggest that both these models (homopolymer and heteropolymer) could be applicable depending on the solution conditions. For example, the formation of secondary structure and compact state is not simultaneous in aqueous buffer. In aqueous buffer, formation of the compact state occurs through a two-state co-operative transition following heteropolymer formalism, whereas secondary structure formation takes place gradually. In contrast, in the presence of urea, a compaction of the protein radius occurs gradually over an extended range of salt concentration following homopolymer formalism. The salt-induced compaction and the formation of secondary structure take place simultaneously in the presence of urea.
Figures

Similar articles
-
The slow folding reaction of barstar: the core tryptophan region attains tight packing before substantial secondary and tertiary structure formation and final compaction of the polypeptide chain.J Mol Biol. 2000 Sep 15;302(2):479-95. doi: 10.1006/jmbi.2000.4060. J Mol Biol. 2000. PMID: 10970747
-
Perchlorate-induced formation of the alpha-helical structure of mastoparan.J Biochem. 1994 Oct;116(4):910-5. doi: 10.1093/oxfordjournals.jbchem.a124615. J Biochem. 1994. PMID: 7883768
-
Studies of early events of folding of a predominately β-sheet protein using fluorescence correlation spectroscopy and other biophysical methods.Biochemistry. 2014 Mar 11;53(9):1393-402. doi: 10.1021/bi4014837. Epub 2014 Feb 27. Biochemistry. 2014. PMID: 24555410
-
How fast is protein hydrophobic collapse?Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12117-22. doi: 10.1073/pnas.2033863100. Epub 2003 Oct 6. Proc Natl Acad Sci U S A. 2003. PMID: 14530404 Free PMC article.
-
How, when and why proteins collapse: the relation to folding.Curr Opin Struct Biol. 2012 Feb;22(1):14-20. doi: 10.1016/j.sbi.2011.10.005. Epub 2011 Nov 19. Curr Opin Struct Biol. 2012. PMID: 22104965 Free PMC article. Review.
Cited by
-
The presence of non-native helical structure in the unfolding of a beta-sheet protein MPT63.Protein Sci. 2017 Mar;26(3):536-549. doi: 10.1002/pro.3103. Epub 2017 Feb 12. Protein Sci. 2017. PMID: 27997710 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

