Aptamer-functionalized nano-biosensors
- PMID: 22303178
- PMCID: PMC3267226
- DOI: 10.3390/s91210356
Aptamer-functionalized nano-biosensors
Abstract
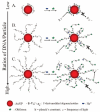
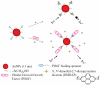
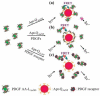
Nanomaterials have become one of the most interesting sensing materials because of their unique size- and shape-dependent optical properties, high surface energy and surface-to-volume ratio, and tunable surface properties. Aptamers are oligonucleotides that can bind their target ligands with high affinity. The use of nanomaterials that are bioconjugated with aptamers for selective and sensitive detection of analytes such as small molecules, metal ions, proteins, and cells has been demonstrated. This review focuses on recent progress in the development of biosensors by integrating functional aptamers with different types of nanomaterials, including quantum dots, magnetic nanoparticles (NPs), metallic NPs, and carbon nanotubes. Colorimetry, fluorescence, electrochemistry, surface plasmon resonance, surface-enhanced Raman scattering, and magnetic resonance imaging are common detection modes for a broad range of analytes with high sensitivity and selectivity when using aptamer bioconjugated nanomaterials (Apt-NMs). We highlight the important roles that the size and concentration of nanomaterials, the secondary structure and density of aptamers, and the multivalent interactions play in determining the specificity and sensitivity of the nanosensors towards analytes. Advantages and disadvantages of the Apt-NMs for bioapplications are focused.
Keywords: aptamers; biosensors; cells; metal ions; nanomaterials; proteins.
Figures







Similar articles
-
[Preparation of dual-functional composite magnetic nanomaterials modified with different metals/aptamers and their performance in exosome enrichment].Se Pu. 2021 Oct;39(10):1128-1136. doi: 10.3724/SP.J.1123.2021.06012. Se Pu. 2021. PMID: 34505435 Free PMC article. Chinese.
-
Functional DNA directed assembly of nanomaterials for biosensing.J Mater Chem. 2009 Apr 7;19(13):10.1039/B813939C. doi: 10.1039/B813939C. J Mater Chem. 2009. PMID: 24307758 Free PMC article.
-
DNA functionalized gold nanoparticles for bioanalysis.Anal Methods. 2009 Sep 28;1(1):14-24. doi: 10.1039/b9ay00036d. Anal Methods. 2009. PMID: 32938137 Review.
-
A review of aptamer-conjugated nanomaterials for analytical sample preparation: Classification according to the utilized nanomaterials.Anal Chim Acta. 2024 Jan 25;1287:342001. doi: 10.1016/j.aca.2023.342001. Epub 2023 Nov 7. Anal Chim Acta. 2024. PMID: 38182359 Review.
-
Nanomaterial-assisted aptamers for optical sensing.Biosens Bioelectron. 2010 Apr 15;25(8):1859-68. doi: 10.1016/j.bios.2009.11.012. Epub 2009 Nov 17. Biosens Bioelectron. 2010. PMID: 20129770 Review.
Cited by
-
Graphene and other nanomaterial-based electrochemical aptasensors.Biosensors (Basel). 2012 Jan 13;2(1):1-14. doi: 10.3390/bios2010001. Biosensors (Basel). 2012. PMID: 25585628 Free PMC article. Review.
-
Cancer active targeting by nanoparticles: a comprehensive review of literature.J Cancer Res Clin Oncol. 2015 May;141(5):769-84. doi: 10.1007/s00432-014-1767-3. Epub 2014 Jul 9. J Cancer Res Clin Oncol. 2015. PMID: 25005786 Free PMC article. Review.
-
An aptasensor using ceria electrodeposited-screen-printed carbon electrode for detection of epithelial sodium channel protein as a hypertension biomarker.R Soc Open Sci. 2021 Feb 17;8(2):202040. doi: 10.1098/rsos.202040. R Soc Open Sci. 2021. PMID: 33972878 Free PMC article.
-
Aptamer based fluorometric determination of ATP by exploiting the FRET between carbon dots and graphene oxide.Mikrochim Acta. 2018 Jan 29;185(2):144. doi: 10.1007/s00604-018-2683-z. Mikrochim Acta. 2018. PMID: 29594479
-
An Apta-Biosensor for Colon Cancer Diagnostics.Sensors (Basel). 2015 Sep 3;15(9):22291-303. doi: 10.3390/s150922291. Sensors (Basel). 2015. PMID: 26404293 Free PMC article.
References
-
- Tombelli S., Minunni M., Mascini M. Aptamers-based assays for diagnostics, environmental and food analysis. Biomol. Eng. 2007;24:191–220. - PubMed
-
- Famulok M., Hartig J.S., Mayer G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem. Rev. 2007;107:3715–3743. - PubMed
-
- Mairal T., Özalp V.C., Sánchez P.L., Mir M., Katakis I., O'Sullivan C.K. Aptamers: molecular tools for analytical applications. Anal. Bioanal. Chem. 2008;390:989–1007. - PubMed
LinkOut - more resources
Full Text Sources

