Arabidopsis and the genetic potential for the phytoremediation of toxic elemental and organic pollutants
- PMID: 22303204
- PMCID: PMC3243353
- DOI: 10.1199/tab.0032
Arabidopsis and the genetic potential for the phytoremediation of toxic elemental and organic pollutants
Abstract
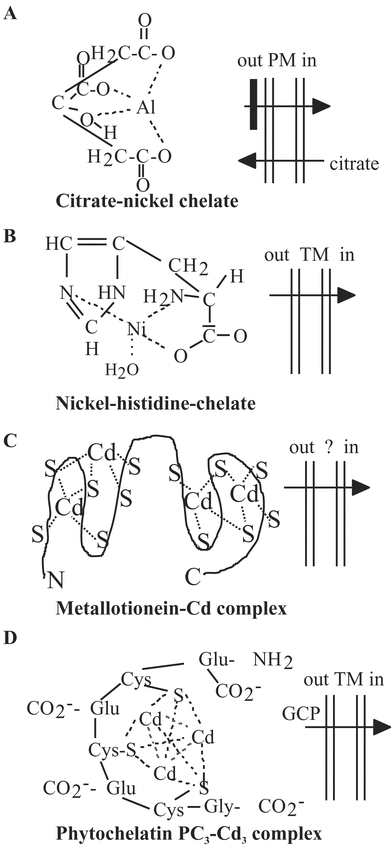
In a process called phytoremediation, plants can be used to extract, detoxify, and/or sequester toxic pollutants from soil, water, and air. Phytoremediation may become an essential tool in cleaning the environment and reducing human and animal exposure to potential carcinogens and other toxins. Arabidopsis has provided useful information about the genetic, physiological, and biochemical mechanisms behind phytoremediation, and it is an excellent model genetic organism to test foreign gene expression. This review focuses on Arabidopsis studies concerning: 1) the remediation of elemental pollutants; 2) the remediation of organic pollutants; and 3) the phytoremediation genome. Elemental pollutants include heavy metals and metalloids (e.g., mercury, lead, cadmium, arsenic) that are immutable. The general goal of phytoremediation is to extract, detoxify, and hyperaccumulate elemental pollutants in above-ground plant tissues for later harvest. A few dozen Arabidopsis genes and proteins that play direct roles in the remediation of elemental pollutants are discussed. Organic pollutants include toxic chemicals such as benzene, benzo(a)pyrene, polychlorinated biphenyls, trichloroethylene, trinitrotoluene, and dichlorodiphenyltrichloroethane. Phytoremediation of organic pollutants is focused on their complete mineralization to harmless products, however, less is known about the potential of plants to act on complex organic chemicals. A preliminary survey of the Arabidopsis genome suggests that as many as 700 genes encode proteins that have the capacity to act directly on environmental pollutants or could be modified to do so. The potential of the phytoremediation proteome to be used to reduce human exposure to toxic pollutants appears to be enormous and untapped.
Keywords: carcinogenic; genetics; heavy metals; hyperaccumulation; metalloids; mineralization; organics; plants; remediation; teratogenic; toxins; transformation; transport.
Figures

References
-
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990;2151(1):403–10. - PubMed
-
- Ames B. N. The detection of environmental mutagens and potential carcinogens. Cancer. 1984;531(1):2034–40. - PubMed
-
- Ames B. N., McCann J., Yamasaki E. Proceedings: carcinogens are mutagens: a simple test system. Mutat Res. 1975;331(1):27–8. - PubMed
-
- Anderson T. A., Guthrie E. A., Walton B. T. Bioremediation in the rhizosphere: plant roots and associated microbes clean contaminated soil. Environ. Sci. & Technol. 1993;271(1):2630–2636.
-
- Anderson T. A., Walton B. T. Comparative fate of [14C] trichoroethylene in the root zone of plants from a former solvent disposal site. Environ. Toxicol. & Chem. 1996;121(1):2041–2047.
LinkOut - more resources
Full Text Sources
