The Arabidopsis thaliana-pseudomonas syringae interaction
- PMID: 22303207
- PMCID: PMC3243347
- DOI: 10.1199/tab.0039
The Arabidopsis thaliana-pseudomonas syringae interaction
Figures
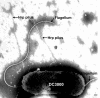


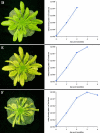



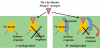








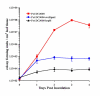

References
-
- Agrios G. N. Plant Pathology. 1997. (San Diego: Academic Press)
-
- Alfano J. R., Kim H-S., Delaney T. P., Collmer A. Evidence that the Pseudomonas syringae pv. syringae hrp-linked hrmA gene encodes an Avr-like protein that acts in an hrp-dependent manner within tobacco cells. Mol. Plant-Microbe Interact. 1997;101(1):580–588. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
