Storage reserve accumulation in Arabidopsis: metabolic and developmental control of seed filling
- PMID: 22303238
- PMCID: PMC3243342
- DOI: 10.1199/tab.0113
Storage reserve accumulation in Arabidopsis: metabolic and developmental control of seed filling
Abstract
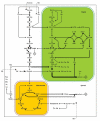
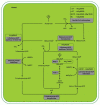
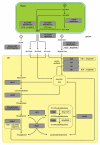
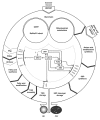
In the life cycle of higher plants, seed development is a key process connecting two distinct sporophytic generations. Seed development can be divided into embryo morphogenesis and seed maturation. An essential metabolic function of maturing seeds is the deposition of storage compounds that are mobilised to fuel post-germinative seedling growth. Given the importance of seeds for food and animal feed and considering the tremendous interest in using seed storage products as sustainable industrial feedstocks to replace diminishing fossil reserves, understanding the metabolic and developmental control of seed filling constitutes a major focus of plant research. Arabidopsis thaliana is an oilseed species closely related to the agronomically important Brassica oilseed crops. The main storage compounds accumulated in seeds of A. thaliana consist of oil stored as triacylglycerols (TAGs) and seed storage proteins (SSPs). Extensive tools developed for the molecular dissection of A. thaliana development and metabolism together with analytical and cytological procedures adapted for very small seeds have led to a good description of the biochemical pathways producing storage compounds. In recent years, studies using these tools have shed new light on the intricate regulatory network controlling the seed maturation process. This network involves sugar and hormone signalling together with a set of developmentally regulated transcription factors. Although much remains to be elucidated, the framework of the regulatory system controlling seed filling is coming into focus.
Keywords: Arabidopsis; seed maturation; seed storage proteins (SSPs); triacylglycerols (TAGs).
Figures


References
-
- Alban C., Job D., Douce R. Biotin metabolism in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;516(1):17–47. - PubMed
-
- Altenbach S. B., Kuo C. C., Staraci L. C., Pearson K. W., Wainwright C., Georgescu A. Accumulation of Brazil nut albumin in seeds of transgenic canola results in enhanced levels of seed protein methionine. Plant Mol. Biol. 1992;186(1):235–245. - PubMed
LinkOut - more resources
Full Text Sources
